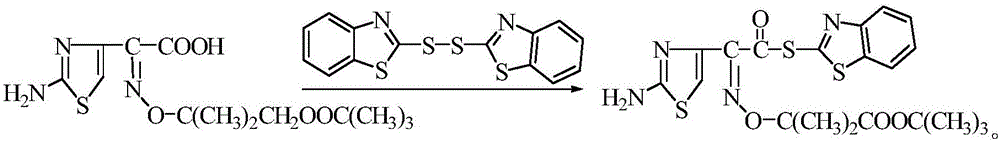
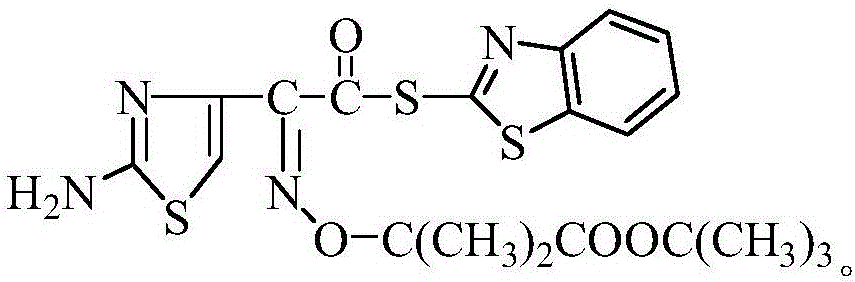
Method for synthesizing 2-Mercaptobenzothiazolyl-(Z)-(2-aminothiazol-4-yl)-2-(tert-butoxycarbonyl) isopropoxyiminoacetate
A technology of ceftazidime side chain acid and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of high price of triphenylphosphine, high production cost, influence on yield and the like, achieve easy mass production, avoid product stickiness, reduce The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] At 20°C, 34g ceftazidime side chain acid and 44gDM were added to the mixed solution of dichloride and acetonitrile (the quality of the mixed solution was 260g, and the density was 0.95g / cm 3 ), after stirring for 10 minutes, add 0.8 mL of pyridine, and dropwise add 7.7 mL of triethylamine at 20°C. The addition process took 1.5 hours. After the dropwise addition was completed, it was incubated at 21±1°C for 4 hours, then cooled to 5°C for 30 minutes, filtered with suction, and dried at 60°C to obtain the active ester of ceftazidime side chain acid, with a yield of 92.3 %, the content is 99.1%.
Embodiment 2
[0031] At 21°C, 35g of ceftazidime side chain acid and 48gDM were added to the mixed solution of dichloride and acetonitrile (the quality of the mixed solution was 250g, and the density was 1.0g / cm 3 ), after stirring for 10 minutes, add 1.0 mL of pyridine, dropwise add 8.0 mL of triethylamine at 22 ° C, after the dropwise addition, raise the temperature to 30 ° C and keep it warm for 50 min, cool down, add 28 mL of triethyl phosphite dropwise at 21 ° C, drop The addition process took 2.5 hours. After the dropwise addition was completed, it was incubated at 21±1°C for 3.0 hours, then cooled to 3°C for 30 minutes, filtered with suction, and dried at 60°C to obtain the active ester of ceftazidime side chain acid. The yield was 92.6%, the content is 99.3%.
Embodiment 3
[0033] At 23°C, 36g of ceftazidime side chain acid and 45gDM were added to the mixed solution of dichloride and acetonitrile (the quality of the mixed solution was 298g, and the density was 1.05g / cm 3 ), after stirring for 10 minutes, add 1.3 mL of pyridine, dropwise add 8.4 mL of triethylamine at 30°C, heat up to 30°C for 50 minutes after the dropwise addition, cool down, add 30mL of triethyl phosphite dropwise at 22°C, drop The addition process took 3.0 hours. After the dropwise addition was completed, it was incubated at 21±1°C for 5.0 hours, then cooled to 0°C and incubated for 30 minutes, filtered with suction, and dried at 60°C to obtain the active ester of ceftazidime side chain acid. The yield was 92.1%, the content is 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com