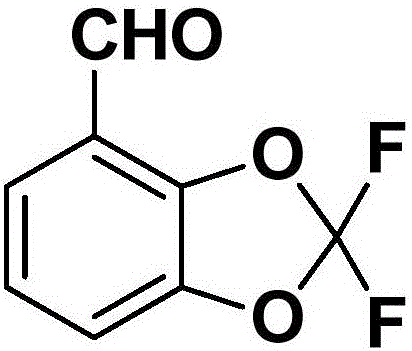
Synthetic method of 2,2-dichloro-1,3-benzodioxole-4-formaldehyde
A technology of benzodioxol and synthesis method, which is applied in the field of synthesis of 2,2-difluoro-1,3-benzodioxol-4-carbaldehyde, can solve the problem that is not suitable for large-scale industrial production and the process is smooth It is beneficial to large-scale industrial production, high yield, and simple post-treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
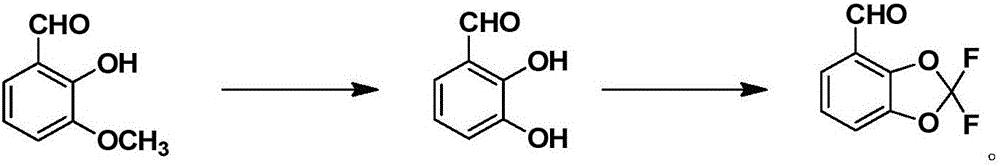
[0032] In a preferred embodiment, the above-mentioned synthetic method comprises the following steps:
[0033] S1: Add 3-methoxy-2-hydroxybenzaldehyde and alkali to a protic solvent, then add hydrogen peroxide dropwise to carry out a hydrolysis reaction; after the reaction is complete, after-treatment, 2,3-dihydroxybenzaldehyde is obtained;
[0034] S2: Add the aqueous solution of 2,3-dihydroxybenzaldehyde and alkali to an aprotic solvent, then add difluorodichloromethane to react; after the reaction is complete, post-treatment, the 2,2-difluoro-1 ,3-Benzodioxol-4-carbaldehyde.
[0035] In a further preferred embodiment, the bases described in S1 and S2 are inorganic bases, and are each independently selected from any of the following: hydroxides of alkali metals, carbonates of alkali metals, acids of alkali metals formula carbonate.
[0036] In a further preferred embodiment, the bases described in S1 and S2 are inorganic bases, and are each independently selected from any ...
Embodiment 1
[0050] Example 1 Synthesis of 2,3-dihydroxybenzaldehyde
[0051]
[0052] In a 2L four-necked flask, add 121.0g (0.80mol) (1.0eq) 3-methoxy-2-hydroxybenzaldehyde, then drop 128g (0.80mol) (1.0eq) of 25% NaOH aqueous solution within 0.5h ), and then added dropwise 940ml (3.5eq) of 10% H 2 o 2 , Control the temperature not to exceed 50°C during the dropwise addition, heat to 60°C after the dropwise addition, keep warm and reflux for 18h. After the reaction is completed, add 10% hydrochloric acid to acidify to pH=1~2, let it stand still, extract with dichloromethane, separate the lower organic phase, remove the dichloromethane by precipitation, collect the distillate at 115~120℃ / 15mmHg by distillation, cool Finally, 84.2 g of a light yellow solid, namely 2,3-dihydroxybenzaldehyde, was obtained with a yield of 85% and mp=104-105°C.
Embodiment 2
[0053] Example 2 Synthesis of 2,3-dihydroxybenzaldehyde
[0054] In a 2L four-necked flask, add 121.0g (0.80mol) (1.0eq) 3-methoxy-2-hydroxybenzaldehyde, then drop 128g (0.80mol) (1.0eq) of 25% NaOH aqueous solution within 0.5h ), and then added dropwise 10% of 671ml (2.5eq) of H 2 o 2 , Control the temperature not to exceed 50°C during the dropwise addition, heat to 60°C after the dropwise addition, keep warm and reflux for 18h. After the reaction is completed, add 10% hydrochloric acid to acidify to pH=1~2, let it stand still, extract with dichloromethane, separate the lower organic phase, remove the dichloromethane by precipitation, collect the distillate at 115~120℃ / 15mmHg by distillation, cool Finally, 66.3 g of a light yellow solid, namely 2,3-dihydroxybenzaldehyde, was obtained, with a yield of 67%, and mp=104-105°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com