Methane sulfonic acid pradaxa pellet and preparation method
A technology for dabigatran etexilate mesylate and gatran etexilate pellets, applied in the field of medicine, can solve the problems of wet dabigatran etexilate mesylate raw material, large increase in substance, reduced curative effect, etc. Stable and reliable, fast drug application, good film-forming effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
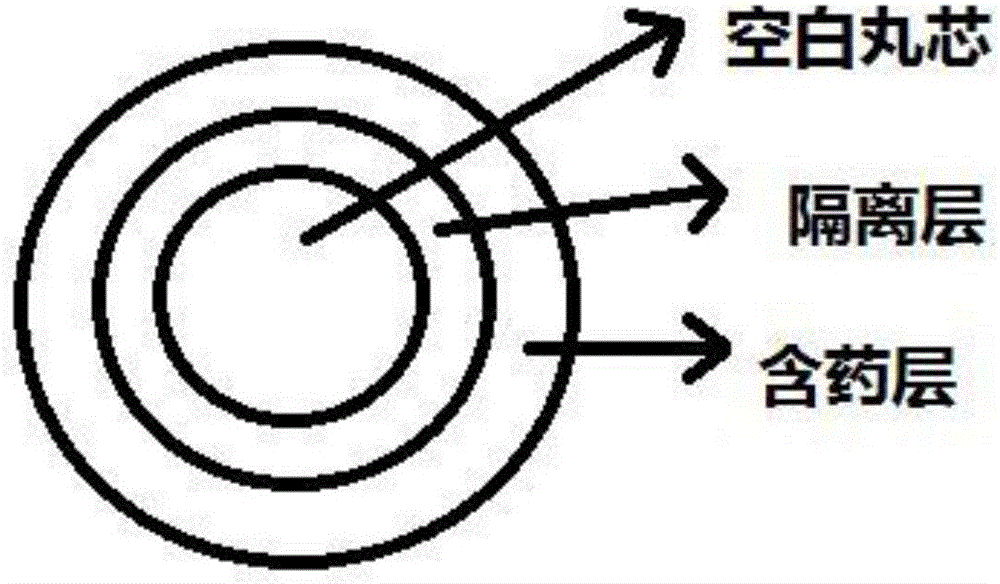
[0054] This embodiment provides a kind of dabigatran etexilate mesylate pellets and preparation method. Dabigatran etexilate mesylate pellet structure schematic diagram of the present invention is as figure 1 shown.
[0055] The structure of dabigatran etexilate mesylate pellets comprises a pellet core, an isolation layer wrapped in the outer layer of the pellet core, and a drug-containing layer wrapped in the outer layer of the isolation layer; wherein the pellet core contains glycine and hypromellose; the isolation layer Contains hypromellose phthalate and talc; drug-containing layer contains dabigatran etexilate mesylate, methyl methacrylate and talc;
[0056] Ball core contains 30% glycine and 5% hypromellose; barrier layer contains 15% hypromellose phthalate and 5% talc; drug-containing layer contains 30% dabigatran mesylate ester, 5% methyl methacrylate and 10% talc.
[0057] The present invention also provides a kind of preparation method of above-mentioned dabigatra...
Embodiment 2
[0065] This embodiment provides a kind of dabigatran etexilate mesylate pellets and preparation method. Dabigatran etexilate mesylate pellet structure schematic diagram of the present invention is as figure 1 shown.
[0066] The structure of dabigatran etexilate mesylate pellets comprises a pellet core, an isolation layer wrapped in the outer layer of the pellet core, and a drug-containing layer wrapped in the outer layer of the isolation layer; wherein the pellet core contains glycine and hypromellose; the isolation layer Contains hypromellose phthalate and talc; drug-containing layer contains dabigatran etexilate mesylate, methyl methacrylate and talc;
[0067] Ball core contains 30% glycine and 1% hypromellose; barrier layer contains 5% hypromellose phthalate and 5% talc; drug-containing layer contains 49% dabigatran mesylate ester, 5% methyl methacrylate and 5% talc.
[0068] The present invention also provides a kind of preparation method of above-mentioned dabigatran ...
Embodiment 3
[0076] This embodiment provides a kind of dabigatran etexilate mesylate pellets and preparation method. Dabigatran etexilate mesylate pellet structure schematic diagram of the present invention is as figure 1 shown.
[0077] The structure of dabigatran etexilate mesylate pellets comprises a pellet core, an isolation layer wrapped in the outer layer of the pellet core, and a drug-containing layer wrapped in the outer layer of the isolation layer; wherein the pellet core contains glycine and hypromellose; the isolation layer Contains hypromellose phthalate and talc; drug-containing layer contains dabigatran etexilate mesylate, methyl methacrylate and talc;
[0078] Ball core contains 32% glycine and 3% hypromellose; barrier layer contains 12% hypromellose phthalate and 8% talc; drug-containing layer contains 32% dabigatran mesylate ester, 7% methyl methacrylate and 6% talc.
[0079] The present invention also provides a kind of preparation method of above-mentioned dabigatran...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com