Application of ginsenoside used as heparanase inhibitor in preparation of tumor therapeutic drug
A technology of heparanase and ginsenoside, which is applied in the direction of antineoplastic drugs, drug combinations, and pharmaceutical formulations, and can solve the complex production process of antibodies and vaccine antagonists, the difficulty of preparing monomer anticoagulant effects, and the production cost advanced questions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Safety Evaluation
[0039] The research results of the non-clinical safety evaluation of the present invention are as follows:
[0040] 1. Oral acute toxicity test in mice
[0041] Under the conditions of the maximum dosage concentration and maximum dosage volume, the mice were orally given ginsenoside Rk1 12g / kgwt., observed continuously for 14 days, and no death or abnormal toxic reaction was found in the animals. It shows that the maximum tolerated dose of ginsenoside Rk1 mice is 12g / kg wt.
[0042] 2. Beagle dog oral acute toxicity test
[0043] Under the conditions of maximum dosage concentration and maximum dosage volume, Beagle dogs were given ginsenoside Rk12g / kgwt. orally by gavage, and were observed continuously for 14 days. No death or abnormal toxic reaction was found in the animals. It shows that the maximum tolerated dose of ginsenoside Rk1Beagle dog gavage is 2g / kg wt.
[0044] 3. Long-term toxicity of intragastric administration in rats
...
Embodiment 2
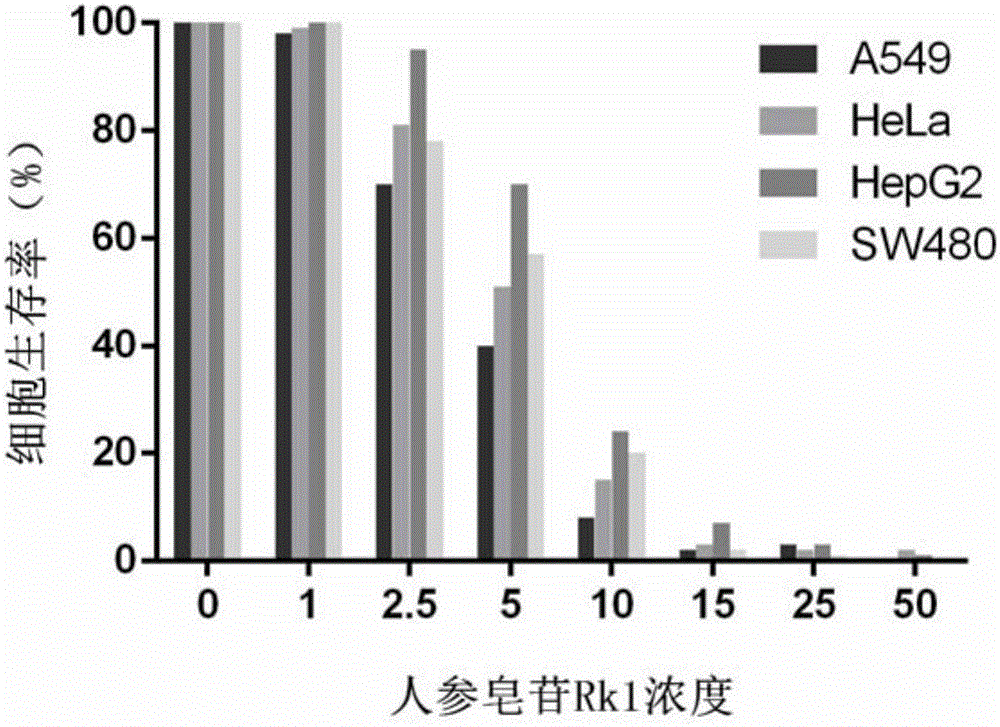
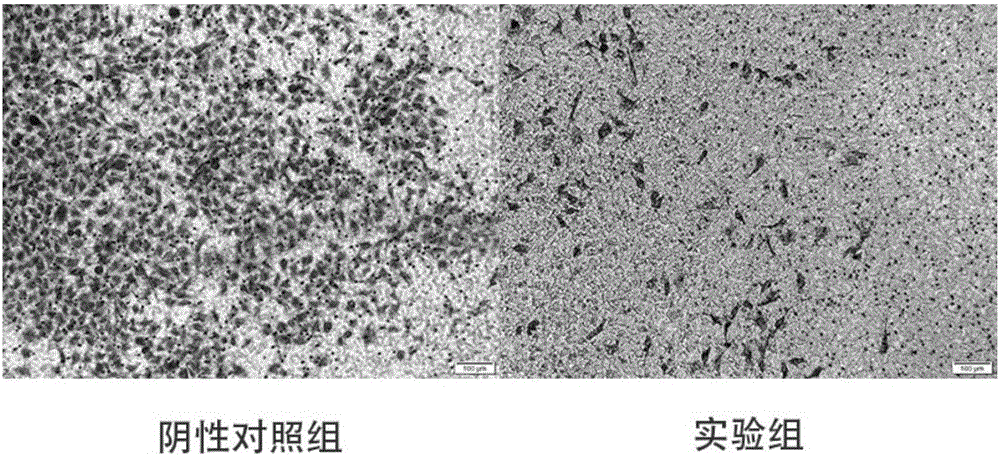
[0052] Embodiment 2 Pharmacodynamics
[0053] Research result of the present invention is as follows:
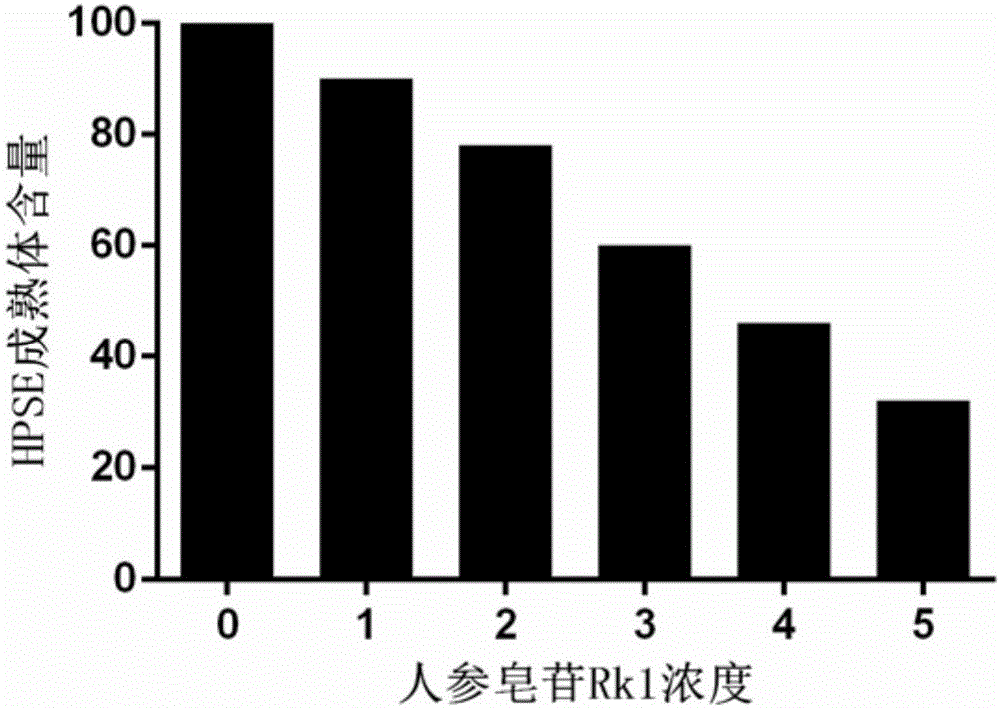
[0054] 1. Ginsenoside Rk1 down-regulates the content of mature heparanase
[0055] Materials: human lung cancer cell A549, heparanase-specific antibody (Santa Cruz), ginsenoside Rk1 (Dalian Meilun Biotechnology Co., Ltd., purity >99%), denaturing polyacrylamide gel electrophoresis system (BioRad).
[0056] method:
[0057] Culture human lung cancer cells A549 to the logarithmic growth phase at 2.0×10 6 Cells / plate were subcultured in 100mm cell culture plate, 37°C, 5% CO 2 Cultivate in the environment for 24 hours, add ginsenoside Rk1 ethanol solution to the medium until the final concentrations of ginsenoside Rk1 are 1, 2, 3, 4, 5 μg / mL (the stock solution is 1 mg / mL ginsenoside Rk1 ethanol solution), 37 °C, 5% CO 2 Continue to culture in the environment for 4 hours, collect the cells, and perform denatured polyacrylamide gel electrophoresis——Western blot detection, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com