Method for removing fluorine and silicon ions from nickel cobalt manganese sulfate solution
A nickel sulfate and silicon ion technology, applied in nickel sulfate, manganese sulfate, cobalt sulfate, etc., can solve the problems of non-metallic impurity removal methods that have not been reported, and achieve easy industrial production, good impurity removal effect, and low production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
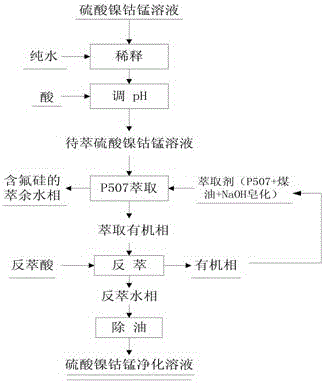
[0031] A Dilution: Take 100L nickel-cobalt-manganese sulfate solution and dilute it with water until the total amount of nickel, cobalt and manganese ions is 30g / L, F- is 2g / L, and Si4+ is 0.2g / L;
[0032] B adjust pH: the diluted nickel-cobalt-manganese sulfate solution is adjusted to pH 4 with sulfuric acid to obtain 200L nickel-cobalt-manganese sulfate solution to be extracted;
[0033] C Extraction: The nickel-cobalt-manganese sulfate solution to be extracted obtained in step B is subjected to multistage countercurrent extraction with 400L P507 and sulfonated kerosene mixed extractant, wherein the mixed extractant is composed of 100L P507 organic phosphorus extractant and 300L sulfonated kerosene. The ratio is 2.0, the pH of the aqueous phase is 4.2, and 150L of the extracted aqueous phase and 450L of the extracted organic phase are obtained by separation;
[0034] D washing: rinse the extracted organic phase obtained in step C with 450L pure water;
[0035] E stripping: ...
Embodiment 2
[0038] A Dilution: Take 100L nickel-cobalt-manganese sulfate solution and dilute it with water until the total amount of nickel, cobalt and manganese ions is 60g / L, F- is 5g / L, and Si4+ is 2g / L;
[0039] B adjust pH: the diluted nickel-cobalt-manganese sulfate solution is adjusted to pH 4 with sulfuric acid to obtain 150L nickel-cobalt-manganese sulfate solution to be extracted;
[0040] C Extraction: The nickel-cobalt-manganese sulfate solution to be extracted obtained in step B is subjected to multistage countercurrent extraction with 630L P507 and sulfonated kerosene mixed extractant, wherein the mixed extractant consists of 157.5 L P507 organic phosphorus extractant and 4725 L sulfonated kerosene Composition, the ratio is 4.2, the pH of the aqueous phase is 6.0, and the extracted aqueous phase is 120L, and the extracted oil phase is 660L;
[0041] D washing: rinse the extracted organic phase obtained in step C with 1320L pure water;
[0042] E stripping: the organic phase...
Embodiment 3
[0045] A Dilution: Take 100L nickel-cobalt-manganese sulfate solution and dilute it with water until the total amount of nickel, cobalt and manganese ions is 50g / L, F- is 4g / L, and Si4+ is 1.5g / L;
[0046] B adjust pH: the diluted nickel-cobalt-manganese sulfate solution is adjusted to pH 5 with sulfuric acid to obtain 180L nickel-cobalt-manganese sulfate solution to be extracted;
[0047] C extraction: the nickel-cobalt-manganese sulfate solution to be extracted obtained in step B is subjected to multistage countercurrent extraction with 180L P507 and sulfonated kerosene mixed extractant, wherein the mixed extractant is composed of 45L P507 organic phosphorus extractant and 135 L sulfonated kerosene, The phase ratio is 1.0, the pH of the water phase is 5.0, and 150L of the extracted water phase and 210L of the extracted oil phase are obtained by separation;
[0048] D washing: rinse the extracted organic phase obtained in step C with 315L pure water;
[0049]E stripping: the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com