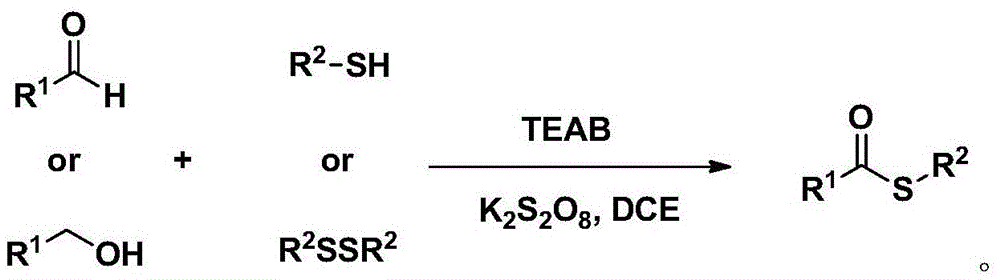
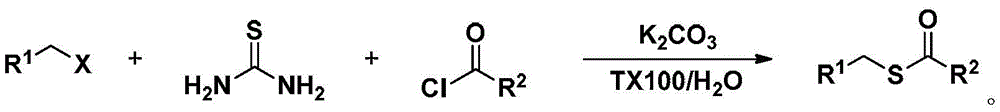
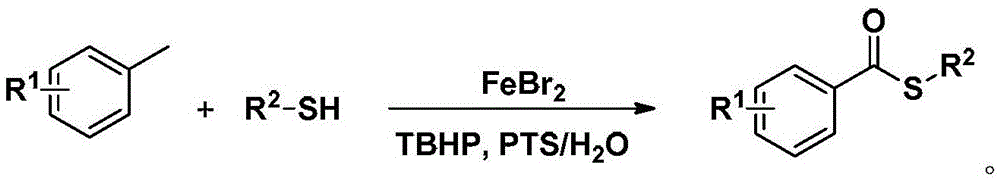
Method for synthesizing S-substituted thiobenzoate derivative by Bunte salt
A technology of benzoic acid thioester and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of high environmental harm, not conforming to the development direction of green chemistry, increased cost of metal catalysts and surfactants, etc., and achieves less three wastes, simple and safe operation. , the preparation process is reasonable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add 11.3g (50mmol) sodium benzylsulfonate, 107mL (1.06mol) benzaldehyde and 27.6mL (0.15mol) di-tert-butyl peroxide into a 250mL vacuum reaction flask, and fill with nitrogen. The reaction was stirred at 135°C for 12h. After the reaction was completed, the reaction solution was diluted with 100 mL of ethyl acetate, washed three times with saturated brine, the organic layer was separated, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The crude product was separated by column chromatography (the eluent was a mixture of ethyl acetate and petroleum ether, the volume ratio of the two was 50:1) to obtain 9.2 g of S-benzylbenzoic acid thioester with a yield of 81%, and NMR characterization Data are as follows:
[0037] 1 H NMR (500MHz, CDCl 3 )δ:4.32(s,2H),7.23-7.26(m,1H),7.30-7.32(m,2H),7.37-7.38(m,2H),7.41-7.45(m,2H),7.54-7.57( m,1H),7.95-7.97(m,2H);
[0038] 13 C NMR (126MHz, CDCl 3 )δ: 33.49(s), 127.44(s), 128.76(s), ...
Embodiment 2
[0040] Add 11.3g (50mmol) sodium benzylsulfonate, 57mL (0.56mol) benzaldehyde and 9.2mL (50mmol) di-tert-butyl peroxide into a 250mL vacuum reaction flask, and fill with nitrogen. The reaction was stirred at 135°C for 10h. After the reaction was completed, the reaction solution was diluted with 100 mL of ethyl acetate, washed three times with saturated brine, the organic layer was separated, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The crude product was separated by column chromatography (the eluent was a mixture of ethyl acetate and petroleum ether with a volume ratio of 50:1) to obtain 5.1 g of S-benzylbenzoic acid thioester with a yield of 45%.
Embodiment 3
[0042] Add 11.3g (50mmol) sodium benzylsulfonate, 107mL (1.06mol) benzaldehyde and 13.8mL (75mmol) di-tert-butyl peroxide into a 250mL vacuum reaction flask, and fill with nitrogen. The reaction was stirred at 150°C for 8h. After the reaction was completed, the reaction solution was diluted with 100 mL of ethyl acetate, washed three times with saturated brine, the organic layer was separated, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The crude product was separated by column chromatography (the eluent was a mixture of ethyl acetate and petroleum ether, the volume ratio of which was 50:1) to obtain 5.9 g of S-benzylbenzoic acid thioester, with a yield of 52%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com