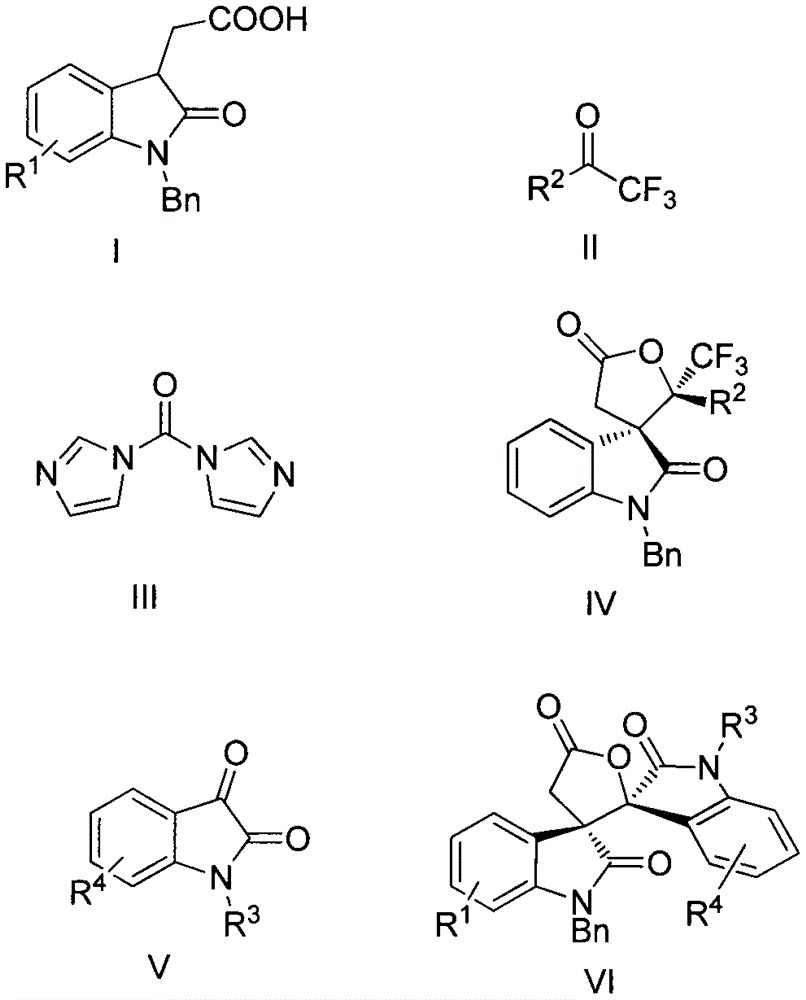
Synthesis method of spiro-oxindole gamma-butyrolactone compound
A technology of spiro-epoxidized indole and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of less synthesis and research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
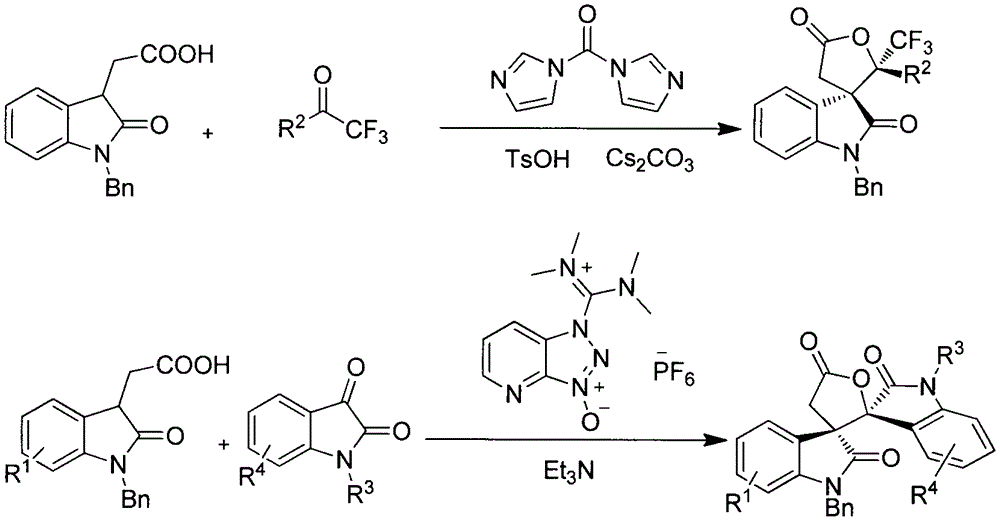
[0018] Example 1: Reaction of 2-(1-benzyl-2-oxoindol-3-yl)acetic acid and trifluoroacetophenone
[0019] 56.2 mg (0.2 mmol) of 2-(1-benzyl-2-oxoindol-3-yl) acetic acid, 52.2 mg (0.3 mmol) of trifluoroacetophenone, N, N'- Carbonyldiimidazole 39mg (0.24mmol), cesium carbonate 130mg (0.4mmol), p-toluenesulfonic acid 34.4mg (0.2mmol) and dichloromethane 2mL were placed in a 25mL two-necked bottle, and reacted at 25°C for 4h. Concentrate, elute through column chromatography with a mixed solvent of petroleum ether:ethyl acetate ratio of 5:1 as the eluent, collect the eluate portion of all products detected, and obtain 70.8 mg of the product after rotary evaporation to remove the solvent. Yield 81%, Dr value 6:1.
[0020] White solid, mp: 191-192℃. 1 H NMR (300MHz, CDCl 3 ): δ7.83(m, 1H), 7.62(d, J=6.2Hz, 1H), 7.49-7.30(m, 3H), 7.29-7.15(4H, m), 7.05-6.86(m, 4H), 6.57(m, 1H), 4.75(d, J=15.2Hz, 1H), 4.34(d, J=15.3Hz, 1H), 3.37(d, J=17.3Hz, 1H), 2.93(d, J=17.4 Hz, 1H). 13 C NMR (...
Embodiment 2
[0021] Example 2: the reaction of 2-(1-benzyl-2-oxoindol-3-yl)acetic acid and 2,2,2,4'-tetrafluoroacetophenone
[0022] 56.2 mg (0.2 mmol) of 2-(1-benzyl-2-oxoindol-3-yl) acetic acid, 57.6 mg (0.3 mmol) of 2,2,2,4'-tetrafluoroacetophenone, such as N shown in formula III, N'-carbonyldiimidazole 39mg (0.24mmol), cesium carbonate 130mg (0.4mmol), p-toluenesulfonic acid 34.4mg (0.2mmol) and dichloromethane 2mL are placed in 25mL two-necked bottle, at 25 The reaction solution was reacted at ℃ for 4 h, the reaction solution was concentrated, and eluted by column chromatography using a mixed solvent of petroleum ether:ethyl acetate ratio of 5:1 as the eluent, and the eluate part of all detected products was collected and spun After distilling off the solvent, 86 mg of the product was obtained, the yield was 95%, and the Dr value was 2:1.
[0023] White solid, mp: 209-211℃. 1 H NMR (300MHz, CDCl 3 ): δ8.04-7.67(m, 2H), 7.49(m, 1H), 7.40-7.29(m, 4H), 7.15-7.00(m, 4H), 6.90-6.35(m, 2...
Embodiment 3
[0024] Example 3: Reaction of 2-(1-benzyl-2-oxoindol-3-yl)acetic acid and 4'-bromo-2,2,2-trifluoroacetophenone
[0025] 56.2 mg (0.2 mmol) of 2-(1-benzyl-2-oxoindol-3-yl) acetic acid, 75.5 mg (0.3 mmol) of 4'-bromo-2,2,2-trifluoroacetophenone 39 mg (0.24 mmol) of N, N'-carbonyldiimidazole, 130 mg (0.4 mmol) of cesium carbonate, 34.4 mg (0.2 mmol) of p-toluenesulfonic acid and 2 mL of dichloromethane were placed in a 25 mL two-necked bottle as shown in formula III, Reacted at 25°C for 4h, concentrated the reaction solution, and eluted by column chromatography with a mixed solvent of petroleum ether:ethyl acetate ratio of 5:1 as the eluent, and collected the eluate portion of all detected products , and the solvent was removed by rotary evaporation to obtain 92 mg of the product with a yield of 90% and a Dr value of 3:1.
[0026] White solid, mp: 225-227℃. 1 H NMR (300MHz, CDCl 3): δ7.78-7.58(m, 3H), 7.41(t, J=7.7Hz, 1H), 7.32-7.28(m, 3H), 7.20(t, J=7.7Hz, 1H), 7.00-6.90( m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com