Method for continuous production and preparation of rifampicin from rifamycin S sodium salt
A technology of rifamycin and sodium salt, which is applied in the field of chemical synthesis, can solve the problems of high price and impact on income, and achieve the effects of short reaction time, reduced side reactions, low toxicity and pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
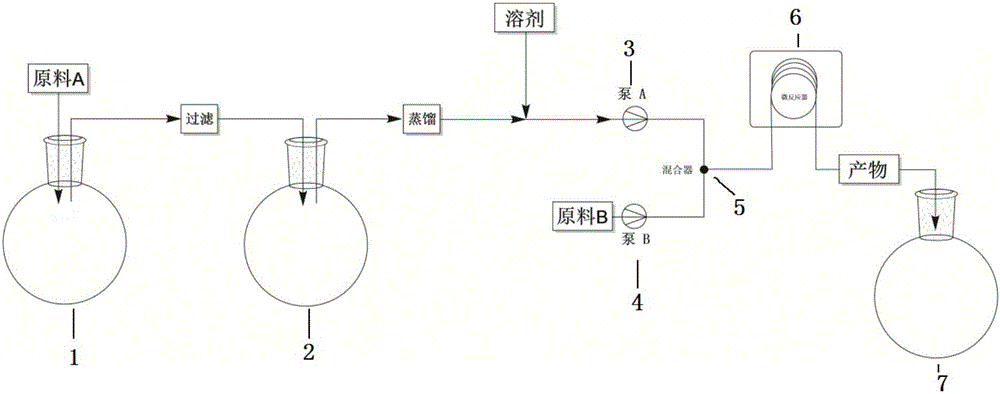
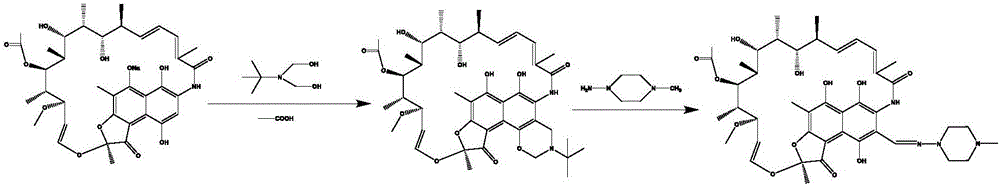
[0029] Take 42g 0.0584mol of rifamycin S sodium salt with a purity of 95% and place it in a three-necked flask A, add 70mL (0.908mol) of N,N-dimethylformamide and 2.84mL of 0.0533mol of sulfuric acid, stir at room temperature for 45min, and then Filter the reaction liquid; after the filtration is completed, put the filtrate in a three-necked beaker bottle B, add 9.86mL (0.0759mol) dimethylol terbutylamine, and stir at 45°C for 2h; After fractional distillation, 70% of the reaction solution was evaporated, then 70mL N,N-dimethylformamide was added and stirred to form a homogeneous solution; then 10mL0.086mol 1-methyl-4-amino-piperazine was placed in three In flask C, add 193mL N,N-dimethylformamide, stir to form a homogeneous solution; pump A to extract the reaction solution in the three-necked flask B at a flow rate of 0.666mL / min, and pump B to directly extract the reaction solution in the three-necked flask C Solution, the flow rate is 1.333mL / min, the two are mixed together...
Embodiment 2
[0031] Take 42g0.0584mol of rifamycin S sodium salt with a purity of 95% and place it in a three-necked flask A, add 70mL (0.908mol) of N,N-dimethylformamide and 1.55mL (0.0292mol) of sulfuric acid, and stir at room temperature for 45min , filter the reaction solution; after the filtration is completed, put the filtrate into a three-necked beaker bottle B, add 9.86mL (0.0759mol) dimethylol terbutylamine, and stir at 45°C for 2h; the reaction solution -0.08 to -0.1mpa, Fractional distillation at 80°C, after distilling off 70% of the reaction solution, add 70mL N,N-dimethylformamide, stir to form a homogeneous solution (0.584mol / l); then add 10mL0.086mol 1-methyl-4- Amino-piperazine was placed in the three-necked flask C, and 193mL N,N-dimethylformamide was added, and stirred to form a homogeneous solution; B directly extracts the solution in the three-necked flask C at a flow rate of 1.333mL / min, mixes the two with a T-shaped mixing valve and pumps them together into the micror...
Embodiment 3
[0033] Take 42g 0.0584mol of rifamycin S sodium salt with a purity of 95% and place it in a three-necked flask A, add 51.3mL (0.666mol) of N,N-dimethylformamide and 2.84mL of 0.0533mol of sulfuric acid, and stir at room temperature for 45min. Filter the reaction liquid; after the filtration is completed, put the filtrate into a three-necked beaker bottle B, add 9.86mL (0.0759mol) dimethylol terbutylamine, and stir at 45°C for 2h; Fractional distillation at ℃, after distilling off 62.5% of the reaction solution, add 70mL N,N-dimethylformamide, stir to form a homogeneous solution; then place 10mL0.086mol 1-methyl-4-amino-piperazine in In the last flask C, add 193mL N,N-dimethylformamide and stir to form a homogeneous solution; the reaction solution in the three-necked flask B is extracted by the pump A at a flow rate of 0.666mL / min, and the pump B directly extracts the reaction solution in the three-necked flask C solution, the flow rate is 1.333mL / min, the two are mixed togethe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com