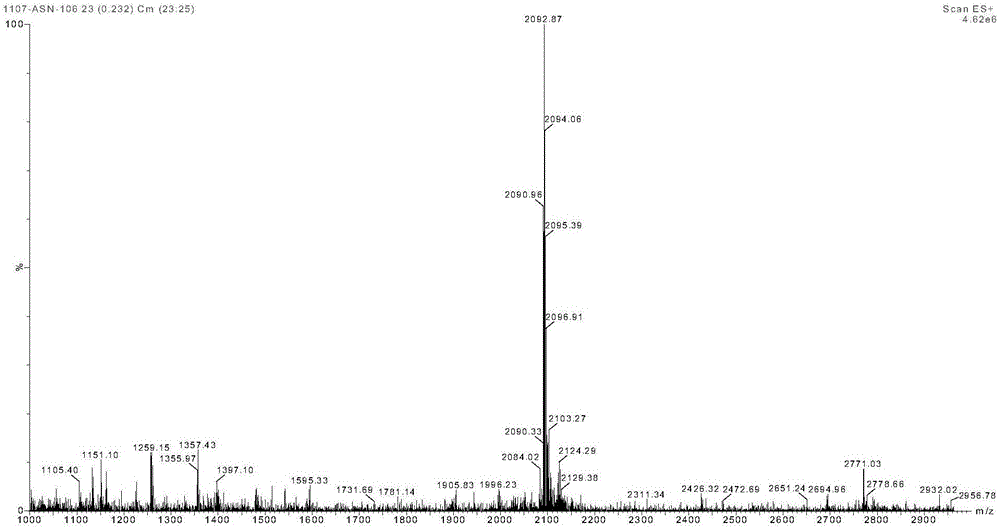
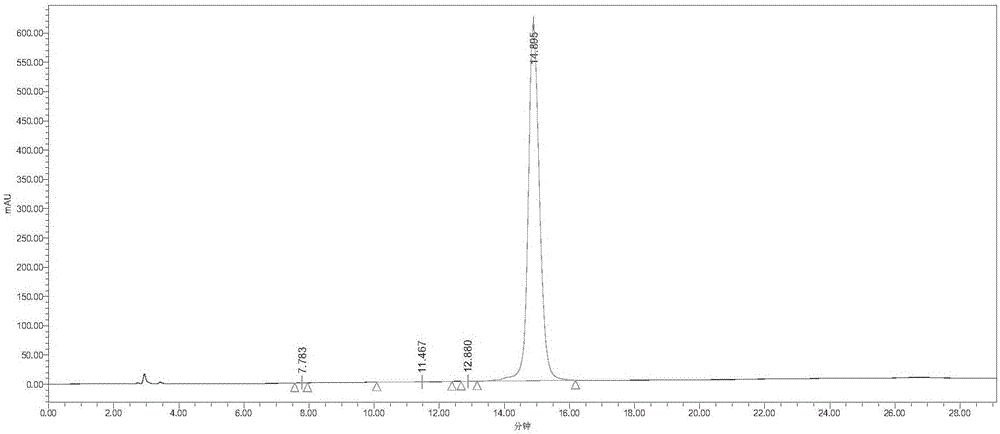
Preparation method and product of exenatide
A technology for exenatide and products, applied in the field of exenatide synthesis by fragment method, which can solve the problems of high cost, high price and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0106] A preparation method of exenatide, the method is synthesized through the protection fragments of the following 13 fragments: His-Gly-COOH, Glu-Gly-Thr-COOH, Phe-Thr-Ser-COOH, Asp-Leu -Ser-COOH, Lys-Gln-COOH, Met-Glu-Glu-COOH, Glu-Ala-Val-COOH, Arg-Leu-Phe-Ile-COOH, Glu-Trp-Leu-COOH, Lys-Asn-Gly -COOH, Gly-Pro-Ser-COOH, Ser-Gly-Ala-COOH and Pro-Pro-Pro-Ser-Rink AmideMBHA Resin.
[0107] In a specific embodiment, the method is synthesized through the following 13 fragments: Fragment 1: Fmoc-His(Trt)-Gly-COOH, Fragment 2: Fmoc-Glu(otBu)-Gly-Thr-COOH, Fragment 3: Fmoc-Phe-Thr-Ser-COOH, Fragment 4: Fmoc-Asp(otBu)-Leu-Ser-COOH, Fragment 5: Fmoc-Lys(Boc)-Gln-COOH, Fragment 6: Fmoc-Met- Glu-Glu-COOH, Fragment 7: Fmoc-Glu(otBu)-Ala-Val-COOH, Fragment 8: Fmoc-Arg(pbf)-Leu-Phe-Ile-COOH, Fragment 9: Fmoc-Glu(otBu)- Trp-Leu-COOH, Fragment 10: Fmoc-Lys(Boc)-Asn-Gly-COOH, Fragment 11: Fmoc-Gly-Pro-Ser-COOH, Fragment 12: Fmoc-Ser(tBu)-Gly-Ala-COOH . Fragment 13: Pro-Pro-Pro-Ser(tBu)...
Embodiment approach
[0110] In a specific embodiment, the method includes the steps of:
[0111] (1) fragments 1-12 were synthesized by liquid phase, and fragment 13 was synthesized by solid phase; and
[0112] (2) Assembling and connecting to obtain the product.
[0113] Wherein, the liquid-phase synthesis method of the fragments 1-12 is to activate the carboxyl terminal of the fragments by N-hydroxysuccinimide and link them with amino acids to extend the amino acid chain.
[0114] The synthesis method of the fragment 1: Fmoc-His(Trt)-Gly-COOH comprises the following steps:
[0115] (1) Synthesis of Fmoc-His(Trt)-COOSu
[0116] Mix Fmoc-His(Trt)-OH, organic solvent and N-hydroxysuccinimide until dissolved, then add dicyclohexylcarbodiimide dissolved in organic solvent for reaction, and obtain Fmoc-His by solid-liquid separation An organic solution of (Trt)-COOSu;
[0117] (2) Synthesis of Fmoc-His(Trt)-Gly-COOH
[0118] Add the mixed aqueous solution of glycine and sodium bicarbonate to the ...
Embodiment 1
[0152] 1. Synthesis of Fmoc-His(Trt)-Gly-COOH
[0153] (1) Synthesis of Fmoc-His(Trt)-COOSu
[0154] Mix 30.99g of Fmoc-His(Trt)-OH with 300ml of 1,4-dioxane, stir at room temperature, add 6.33g of N-hydroxysuccinimide, stir until dissolved, slowly add dicyclohexylcarbodiimide dropwise Mixed solution with 1,4-dioxane at a mass-volume ratio of 11.35g:23mL, reacted at room temperature for 3 hours, filtered under reduced pressure to remove solid insolubles, and obtained 1,4 of Fmoc-His(Trt)-COOSu - dioxane solution.
[0155] (2) Synthesis of Fmoc-His(Trt)-Gly-COOH
[0156] 4.13g of glycine and 4.62g of sodium bicarbonate were dissolved in distilled water, and the resulting solution was added dropwise to the 1,4-dioxane solution of Fmoc-His(Trt)-COOSu obtained in step (1), and stirred at room temperature for 20 hours, concentrated under reduced pressure, adjusted the pH value to 2-3 with a 10% aqueous solution of citric acid, extracted with ethyl acetate, washed the organic pha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com