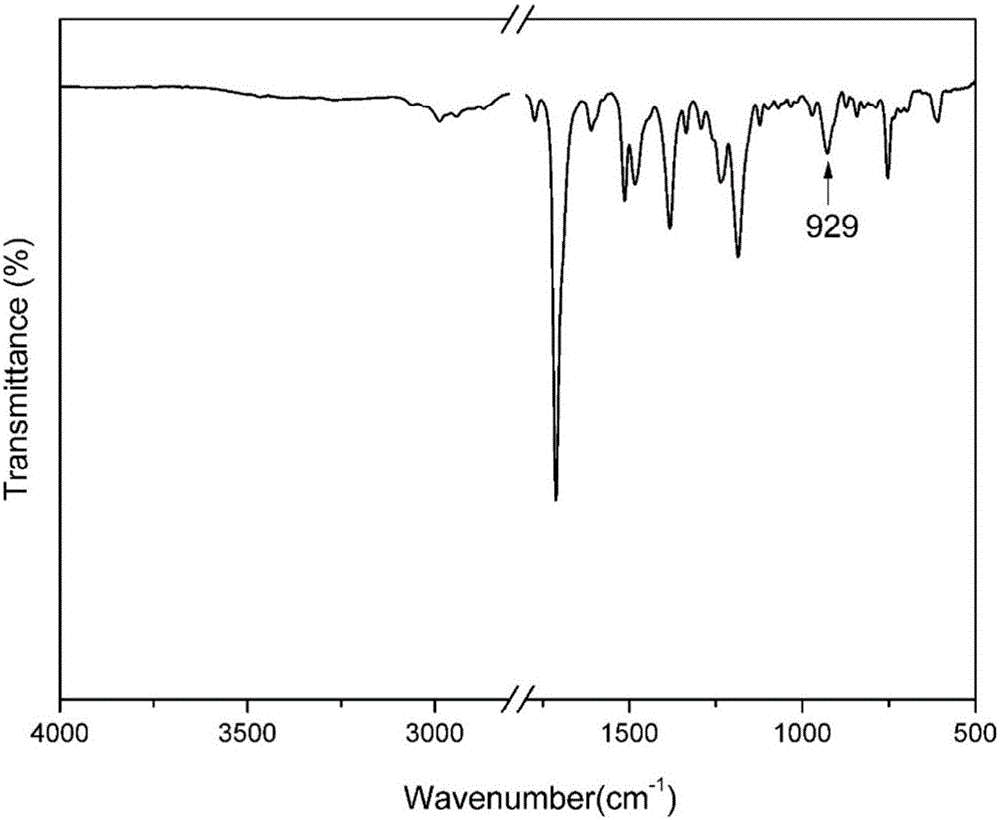
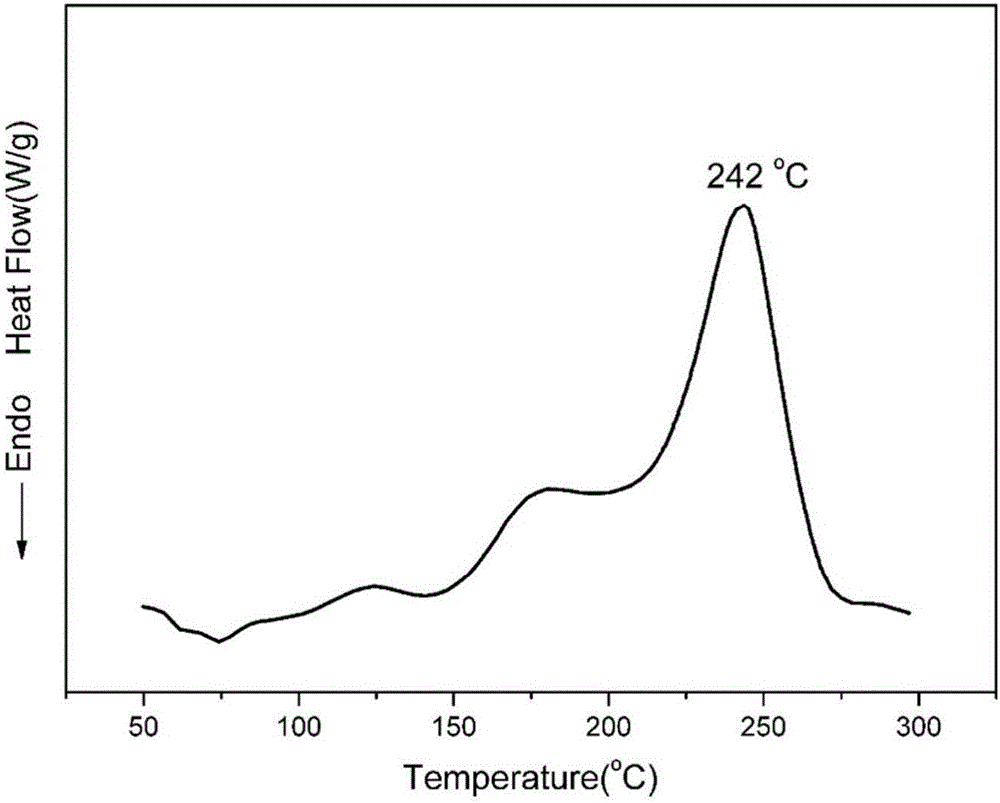
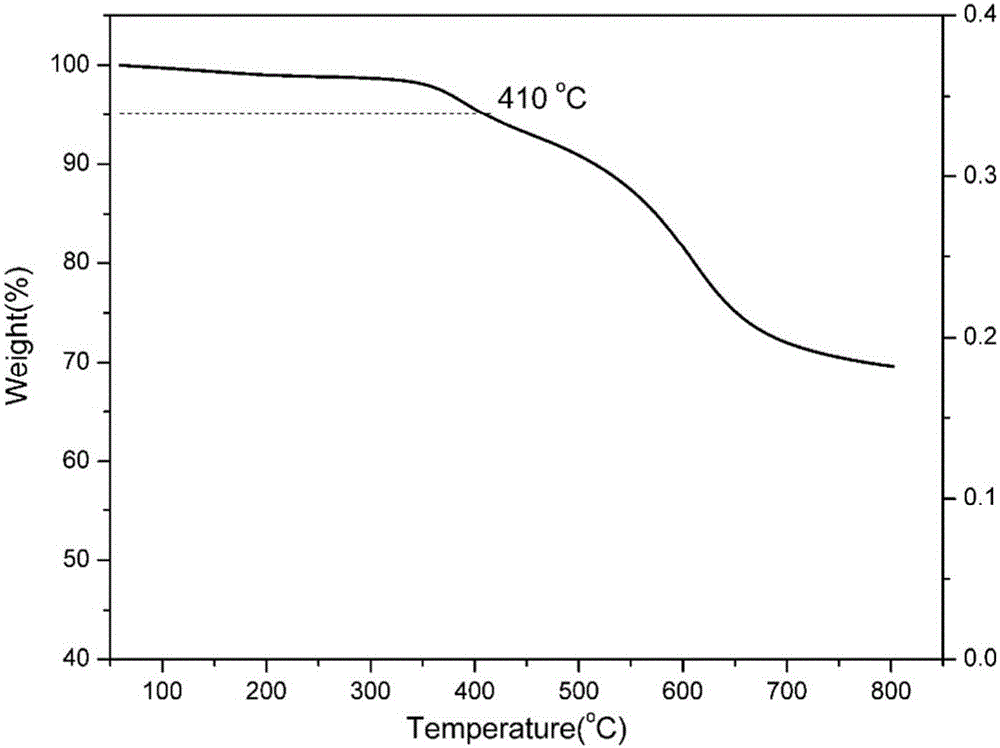
Maleimide terminated type benzoxazine oligomer and preparation method thereof
A maleimide group, maleimide functional technology, applied in the field of benzoxazine oligomer and its preparation, to achieve the effects of simplifying synthesis process, simplifying process and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Add 33.4 g of 2-aminophenol and 30.0 g of maleic anhydride to an ice bath system containing 80 mL of dimethylformamide (DMF), and then add 15 g of phosphorus pentoxide and 8 g of 95- 98% concentrated sulfuric acid, after reacting for 30 minutes, the reaction device was moved to an oil bath at 70° C. for 6 hours. After the reaction, the reaction solution was poured into 200ml of deionized water to obtain a large amount of precipitation. After the reaction solution was washed with deionized water, filtered and dried, 47.6 g of the product maleimide-functionalized phenol was obtained, with a yield of 82%. The reaction equation is as follows:
[0043]
[0044] (2) Weigh 30g of maleimide functionalized phenol obtained in the previous step, and 47.63g of 4,4-dihydroxydiphenylmethane (bisphenol F), 62.89g of 4,4-diaminodiphenylmethane , 38.10 g of paraformaldehyde, and 150 ml of toluene were respectively added to a reaction flask equipped with a stirrer, a thermometer ...
Embodiment 2
[0051] (1) 2-aminophenol in Example 1 is replaced by m-aminophenol, and other steps are the same as those in Example 1.
[0052] Wherein, the structural formula of m-aminophenol is:
[0053] (2) In the second step reaction, the amount of the reactant was changed to: Weigh 30 g of maleimide functionalized phenol obtained in the previous step, and 47.63 g of 4,4-dihydroxydiphenylmethane (bisphenol F) g, 62.89 g of 4,4-diaminodiphenylmethane, 38.10 g of paraformaldehyde, and 150 ml of toluene. 135.65 g of the product was obtained with a yield of 84%.
[0054] In the present embodiment, the structural formula of the obtained benzoxazine oligomer is:
[0055]
[0056] After ring-opening and solidification of the benzoxazine oligomer obtained in this example, its glass transition temperature was measured to be 293° C., its 5% thermal weight loss temperature was 397° C., and its carbon residue rate at 800° C. was 68%.
Embodiment 3
[0058] (1) 2-aminophenol in Example 1 is replaced by m-aminophenol, and other steps are the same as those in Example 1.
[0059] Wherein, the structural formula of p-aminophenol is:
[0060] (2) In the second step reaction, the amount of reactants was changed to: weigh 30 g of maleimide functionalized phenol, and 47.63 g of 4,4-dihydroxydiphenylmethane (bisphenol F), 4, 67.32g of 4-diaminobenzophenone, 38.10g of paraformaldehyde, and 150ml of xylene. 147.67 g of the product was obtained with a yield of 89%.
[0061] In the present embodiment, the structural formula of the obtained benzoxazine oligomer is:
[0062]
[0063] After ring-opening and solidification of the benzoxazine oligomer obtained in this example, its glass transition temperature was measured to be 311° C., its 5% thermal weight loss temperature was 412° C., and its carbon residue rate at 800° C. was 71%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com