New application of tetrahydro isoquinoline alkaloid
A technology of tetrahydroisoquinoline and alkaloids, which is used in drug combination, analysis by chemical reaction of materials, and material analysis by observing the effect on chemical indicators.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis and identification of the screened compounds of the present invention
[0025] All reagents were commercially pure or analytically pure.
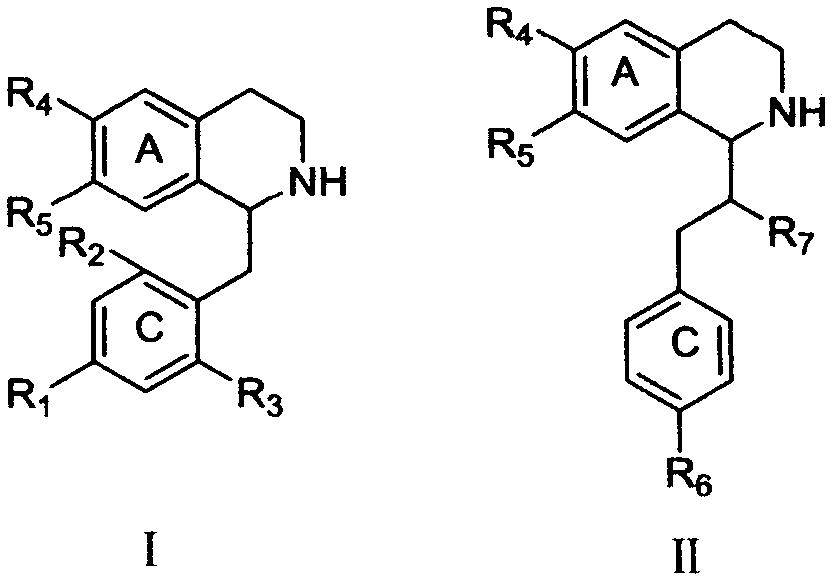
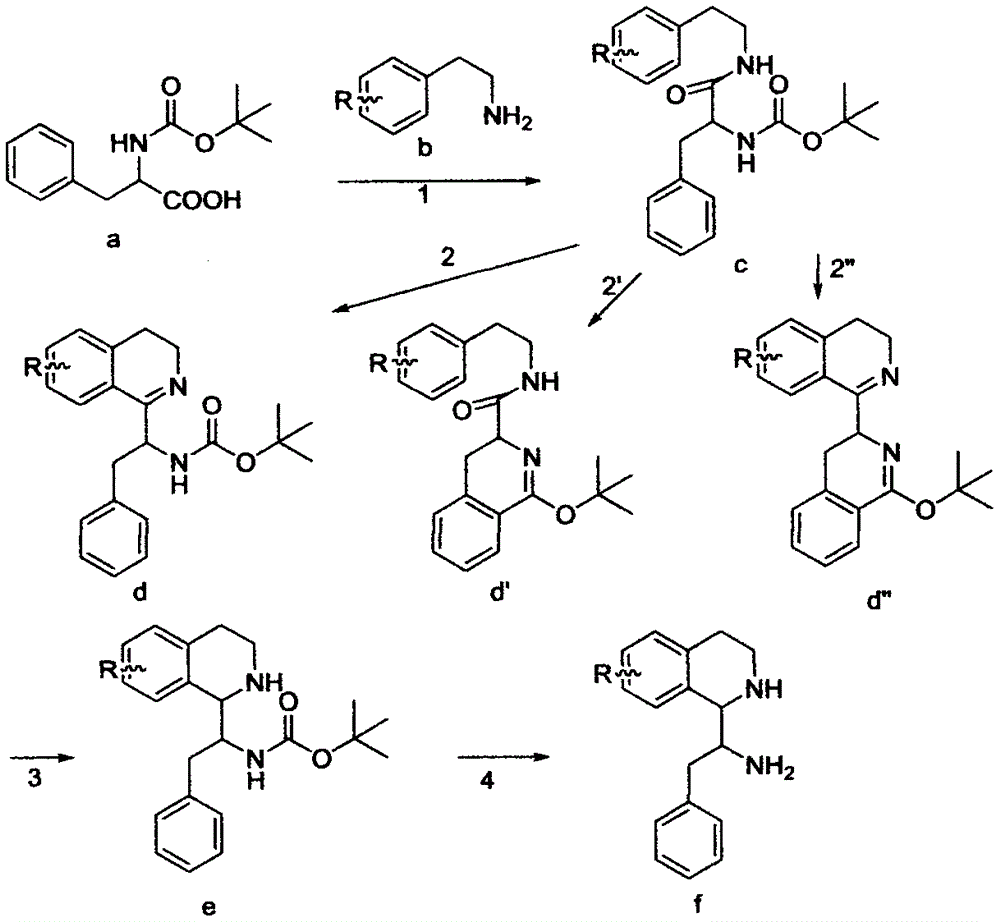
[0026] The basic process of the reaction: the total synthesis of tetrahydroisoquinolines can use phenylethylamine and phenylacetic acid or amino acids as intermediate raw materials for N-acylation condensation, and then obtain dihydroisoquinolines through Bischler-Napieralski reaction, and then After imine reduction and salt-forming reaction, tetrahydroisoquinoline alkaloid salts are finally obtained. The intermediate raw materials can introduce various substituents on the ring by changing the substituent groups on the benzene ring in the phenylethylamine derivatives and phenylacetic acid derivatives or amino acid derivatives.
[0027] (1) Synthesis of intermediate 4-alkoxyphenylacetic acid methyl ester (m1)
[0028] Weigh 0.009mol of methyl p-hydroxyphenylacetate and 0.009mol of anhydrous potassium carbonate, add them to...
Embodiment 2
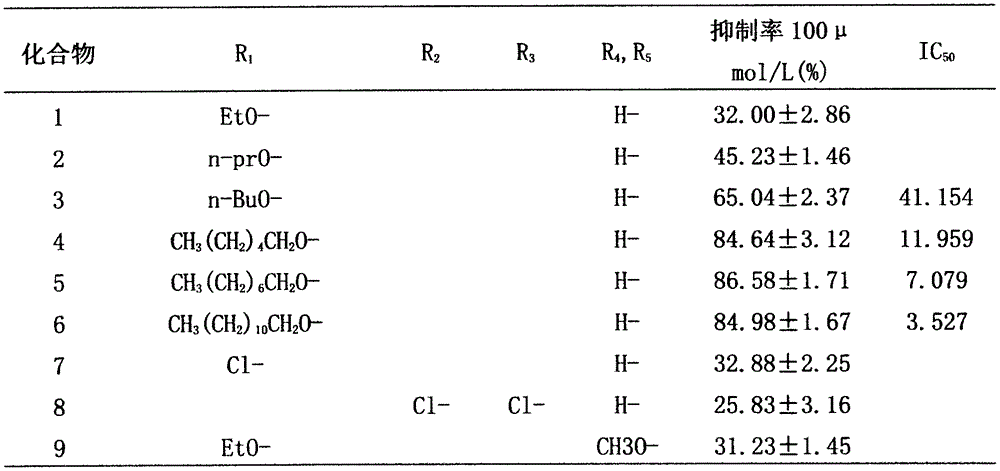
[0076] Determination of α-amylase inhibitory activity of substituted tetrahydroisoquinoline derivatives.
[0077] The measuring instrument is a THERMO Varioskan Flash full-wavelength multifunctional microplate reader.
[0078] Measure the α-amylase inhibitory activity of the compound obtained by the method of Example 1 in the following way:
[0079] Experimental principle: The principle design of the color development between maltose produced by hydrolyzing starch with α-amylase and 3,5-dinitrosalicylic acid (DNS chromogen). Dinitrosalicylic acid (DNS chromogen) undergoes a redox reaction with reducing sugars to generate 3-amino-5-nitrosalicylic acid. The product is brownish red under boiling conditions, and the color is within a certain concentration range. The depth is proportional to the maltose content, and the maltose content is determined by colorimetry.
[0080] Solution preparation: pH 6.9 phosphate buffer solution; soluble starch dissolved in phosphate buffer as sub...
Embodiment 3
[0089] Determination of pancreatic lipase activity of substituted tetrahydroisoquinoline derivatives.
[0090] Measure pancreatic lipase activity with the compound that embodiment 1 method gains with the following method:
[0091] Experimental principle: Pancrelipase can hydrolyze 4-nitrophenyl palmitate (PNPP) to produce yellow 4-nitrophenol (PNP) and palmitic acid. PNP is yellow in aqueous solution, and its maximum absorption wavelength is 405nm, so Pancrelipase activity can be measured by calculating the change in PNP content by measuring the absorbance at 405 nm.
[0092] Solution preparation: the sample compound is first dissolved with a small amount of DMSO, and then diluted to the desired concentration with Tris-HCl buffer. Pancrelipase was made into 1.2 mg / mL enzyme solution with Tris-HCl buffer, and PNPP was first dissolved with a small amount of isopropanol, then diluted with Tris-HCl buffer to make 1 mmol / L substrate solution.
[0093] Each sample consists of 4 sy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com