Method for establishing micro-fluidic chip based human heart model
A microfluidic chip and chip technology, applied in the methods of supporting/immobilizing microorganisms, biochemical equipment and methods, and the determination/inspection of microorganisms, can solve problems such as immaturity in structure and function, and achieve good biocompatibility , for stretch and alignment, maturation and function-promoting effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Establishment of Human Heart Model Using HhiPSC-CM Cells
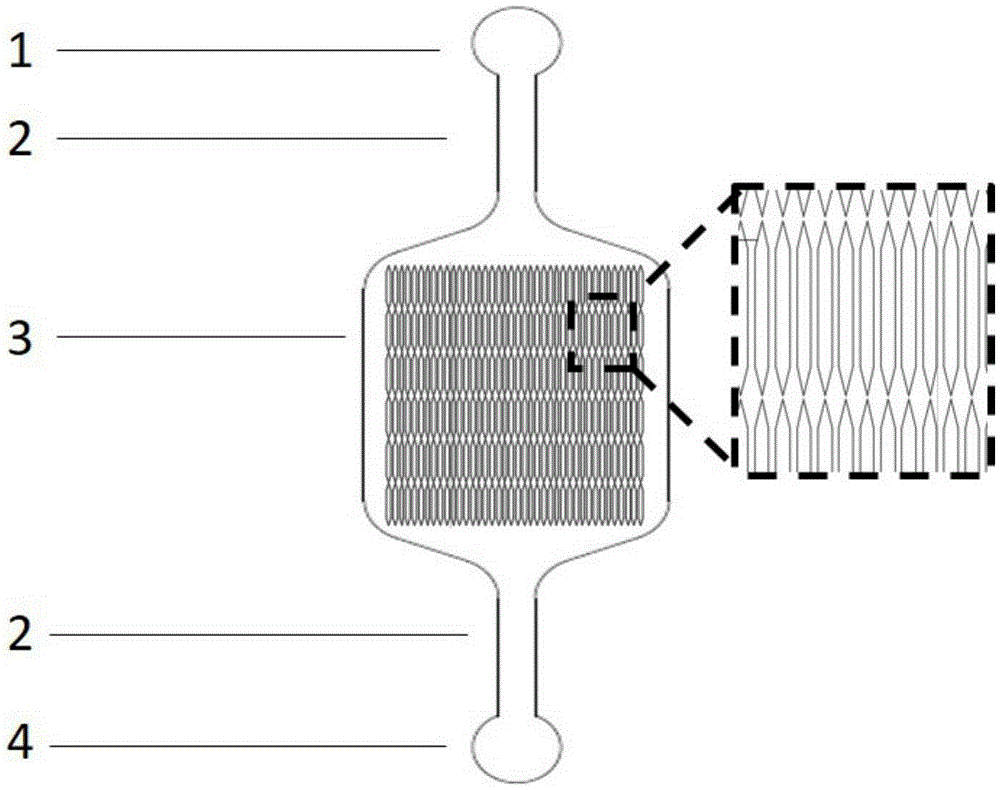
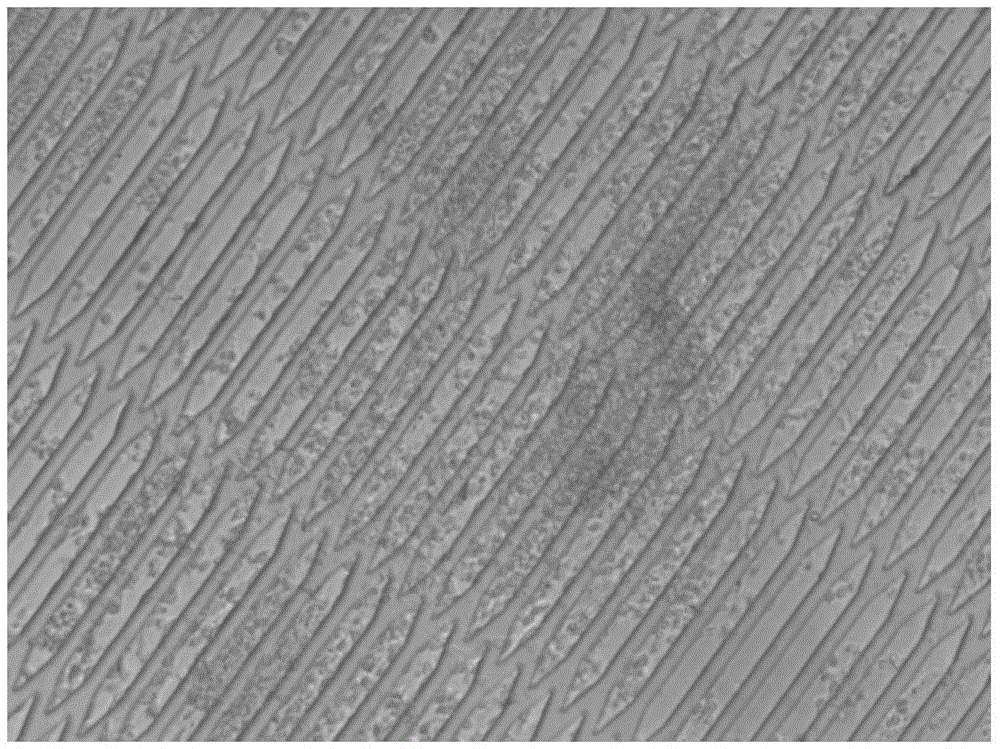
[0040] Using the above microfluidic chip, the structure is as follows figure 1 shown. Prepare Matrigel working solution with a concentration of 150 μg / mL in DMEM-F12 medium, inject it into the chip through the cell inlet pool, let it stand at 4°C overnight, and remove the working solution after 24 hours. After hiPSC-CM cells were digested, diluted to a concentration of 5×10 6 cells / mL cell suspension, add 100 μL of cell suspension into the chip through the cell inlet pool, under the modification of Matrigel, the cells quickly adhere to the wall and evenly spread on the bottom of the cell culture chamber ( figure 2 ), when the uniform distribution of cells in the cell culture chamber was observed under an optical microscope, the chip was immediately moved into a carbon dioxide incubator to continue culturing. After the hiPSC-CM cells were completely attached to the bottom of the cell culture chamber, the cell...
Embodiment 2
[0042] Characterization of functional proteins in human heart model cells
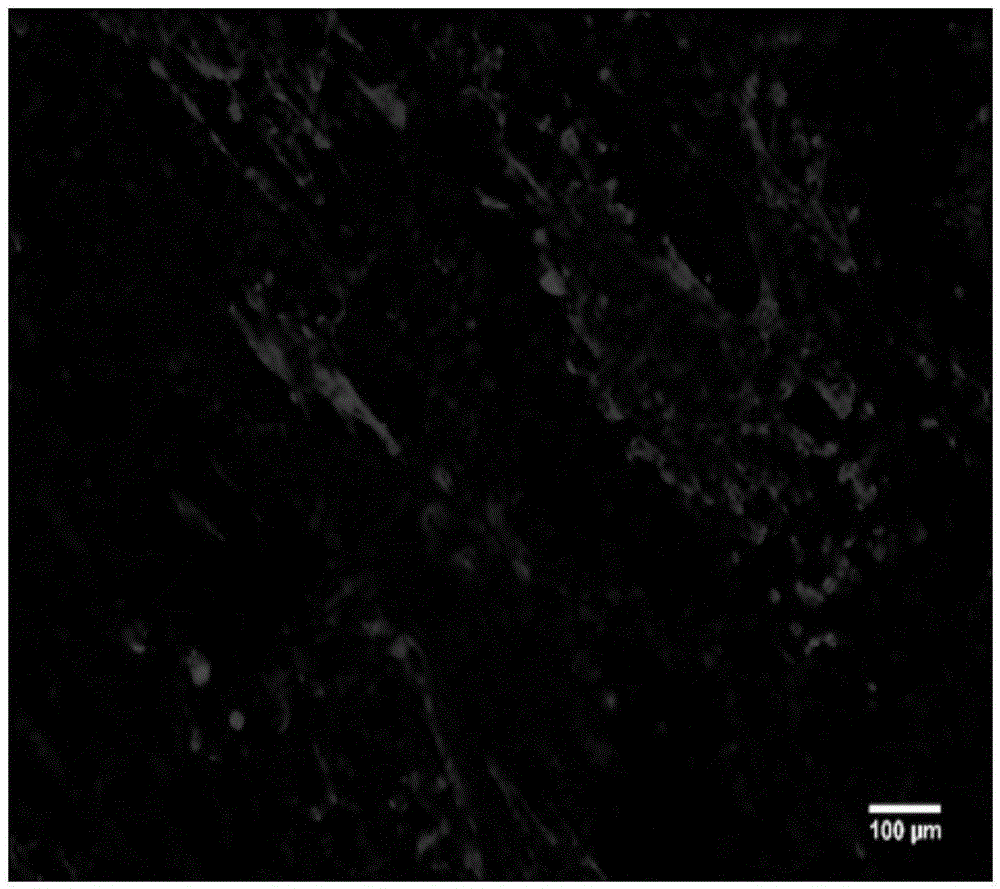
[0043] Using the microfluidic chip designed and manufactured by the laboratory, the structure is as follows: figure 1shown. After chip modification, the same cell inoculation and culture methods as in Example 1 were used to establish a heart model. After 7 days of perfusion, immunofluorescence staining was performed on the cells, and the detected protein was troponin (cTnT). The method is as follows: cells were fixed with 4% paraformaldehyde, washed three times with PBS buffer, 10 min each time; treated with 0.1% triton X-100 porogen for 10 min, washed three times with PBS buffer, 10 min each time; blocked with goat serum for 1 h, The primary antibody (mouse anti-human cTnT) was diluted 1:100, incubated overnight at 4°C, washed three times with PBS buffer for 10 minutes each time; the secondary antibody (AlexaFluor488-labeled goat anti-mouse IgG) was diluted 1:100, incubated at room temperature and p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com