Preparation method of one-dimensional porous carbon nanotube
A technology of porous carbon and nanotubes, applied in the direction of carbon nanotubes, nanocarbons, chemical instruments and methods, etc., can solve the problem of reducing the number of mesopores, and achieve the effect of an environmentally friendly preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
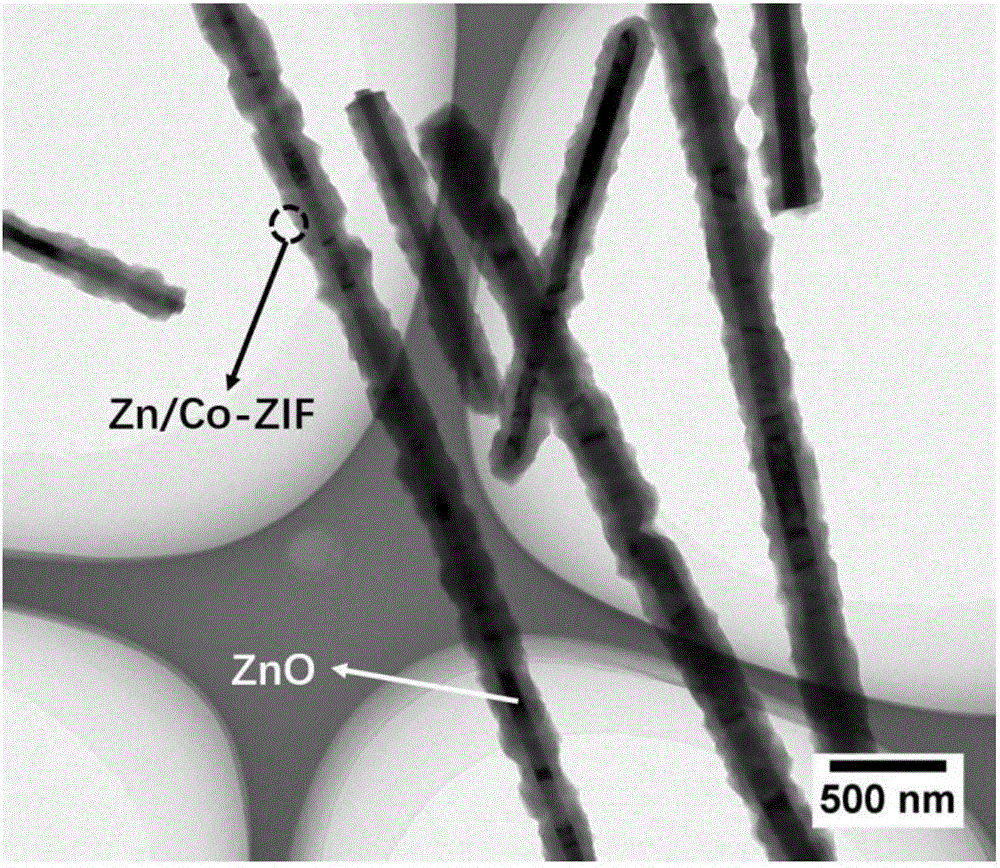
[0043] (1) Take 80.0 mg of ZnO nanowires and add them to an Erlenmeyer flask filled with a mixed solvent of DMF and water (64 mL, volume ratio 3:1), ultrasonicate for 20 min at room temperature to fully diffuse, and then add 0.41 g of 2-methyl For imidazole, after ultrasonication for 2-3min, add 18.0mg of cobalt salt and mix evenly, pour the solution in the conical flask into a hydrothermal kettle, and place it in a 50°C oven to react for 4h. After the reaction, centrifuge, wash and dry , to obtain the ZnO@Zn / Co-ZIF precursor.
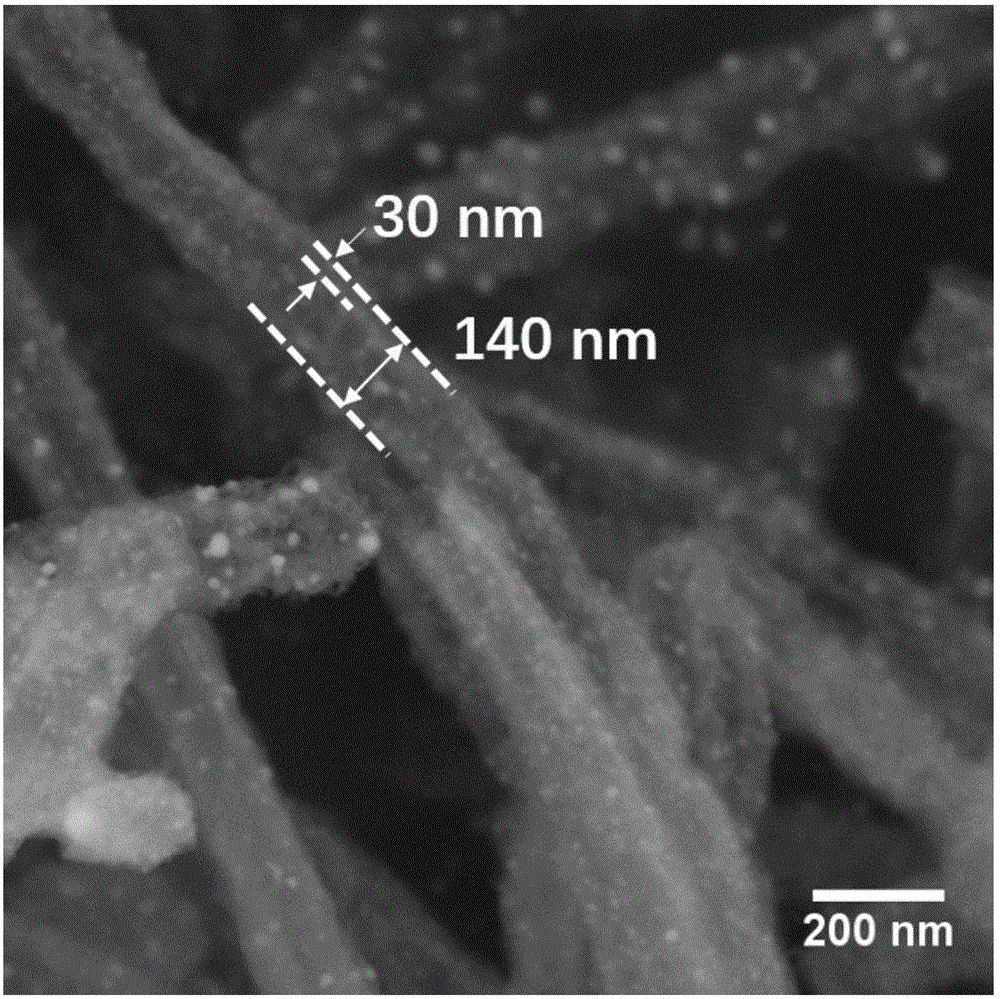
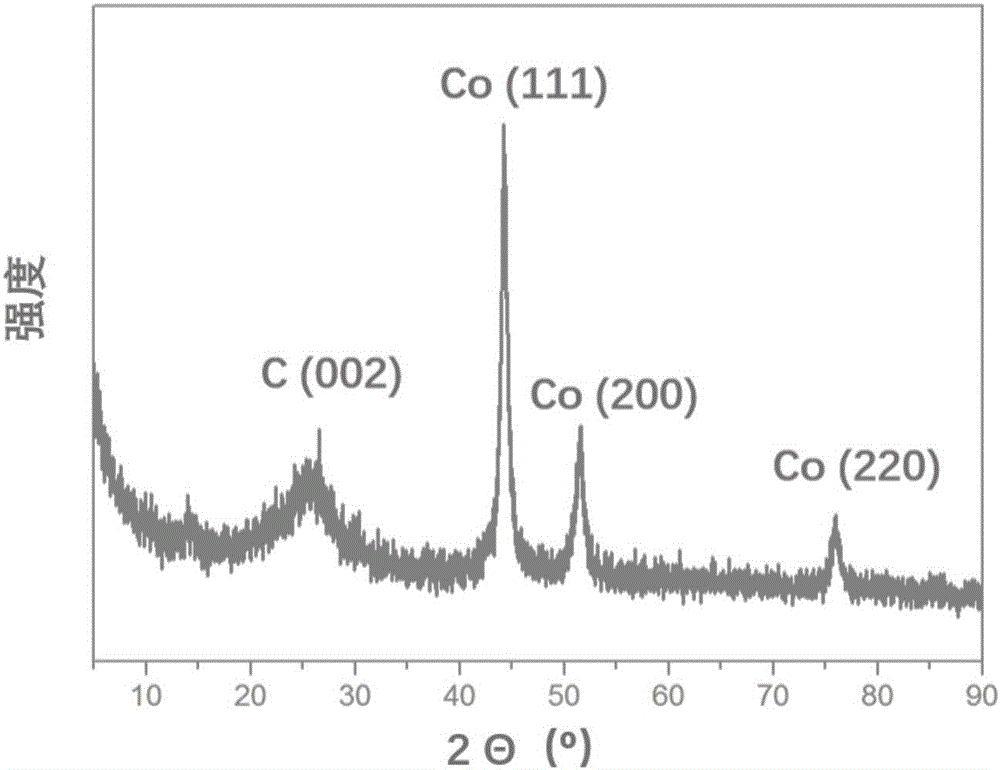
[0044] (2) The ZnO@Zn / Co-ZIF precursor obtained in step 1) was placed in a high-temperature tube furnace, and the temperature was raised to 800 °C at a rate of 5 °C / min in a nitrogen atmosphere with an inert gas flow rate of 50 mL / min. After carbonization at 900° C. for 3 h, it was naturally cooled to room temperature to obtain the nitrogen-cobalt co-doped one-dimensional porous carbon nanotube (Co / NCNT) of the present invention.
[0045] from figure...
Embodiment 2
[0047] (1) Take 80.0 mg of ZnO nanowires and add them to an Erlenmeyer flask filled with a mixed solvent of DMF and water (64 mL, volume ratio 2:1), ultrasonicate for 20 min at room temperature to fully diffuse, and then add 1.6 g of 2-methyl For imidazole, after ultrasonication for 2-3min, add 12.0mg of cobalt salt and mix evenly, pour the solution in the conical flask into a hydrothermal kettle, and place it in an oven at 60°C for 3h. After the reaction, centrifuge, wash and dry , to obtain the ZnO@Zn / Co-ZIF precursor.
[0048] (2) The ZnO@Zn / Co-ZIF precursor obtained in step 1) was placed in a high-temperature tube furnace, and the temperature was raised to 800 °C at a rate of 5 °C / min in a nitrogen atmosphere with an inert gas flow rate of 50 mL / min. After carbonization at 800° C. for 3 h, it was naturally cooled to room temperature to obtain the nitrogen-cobalt co-doped one-dimensional porous carbon nanotube (Co / NCNT) of the present invention.
Embodiment 3
[0050] (1) Take 80.0 mg of ZnO nanowires and add them to an Erlenmeyer flask filled with a mixed solvent of DMF and water (64 mL, volume ratio 3:1). For imidazole, after ultrasonication for 2-3min, add 8.0mg of cobalt salt and mix evenly, pour the solution in the conical flask into a hydrothermal kettle, and place it in an oven at 70°C for 2h. After the reaction, centrifuge, wash and dry , to obtain the ZnO@Zn / Co-ZIF precursor.
[0051] (2) The ZnO@Zn / Co-ZIF precursor obtained in step 1) was placed in a high-temperature tube furnace, and the temperature was raised to 900 °C at a rate of 5 °C / min in a nitrogen atmosphere with an inert gas flow rate of 50 mL / min. After carbonization at 900° C. for 2 h, it was naturally cooled to room temperature to obtain the nitrogen-cobalt co-doped one-dimensional porous carbon nanotube (Co / NCNT) of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com