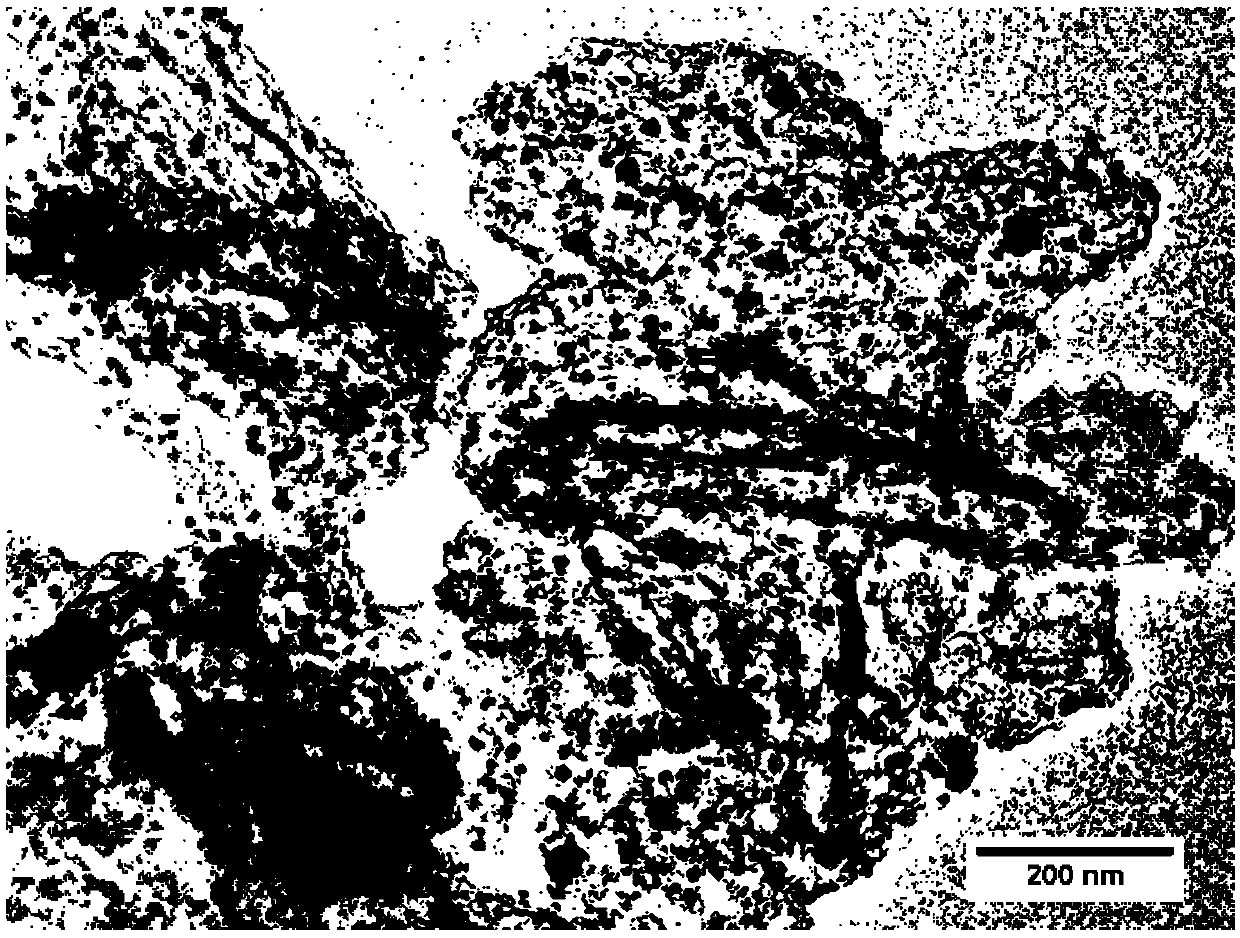
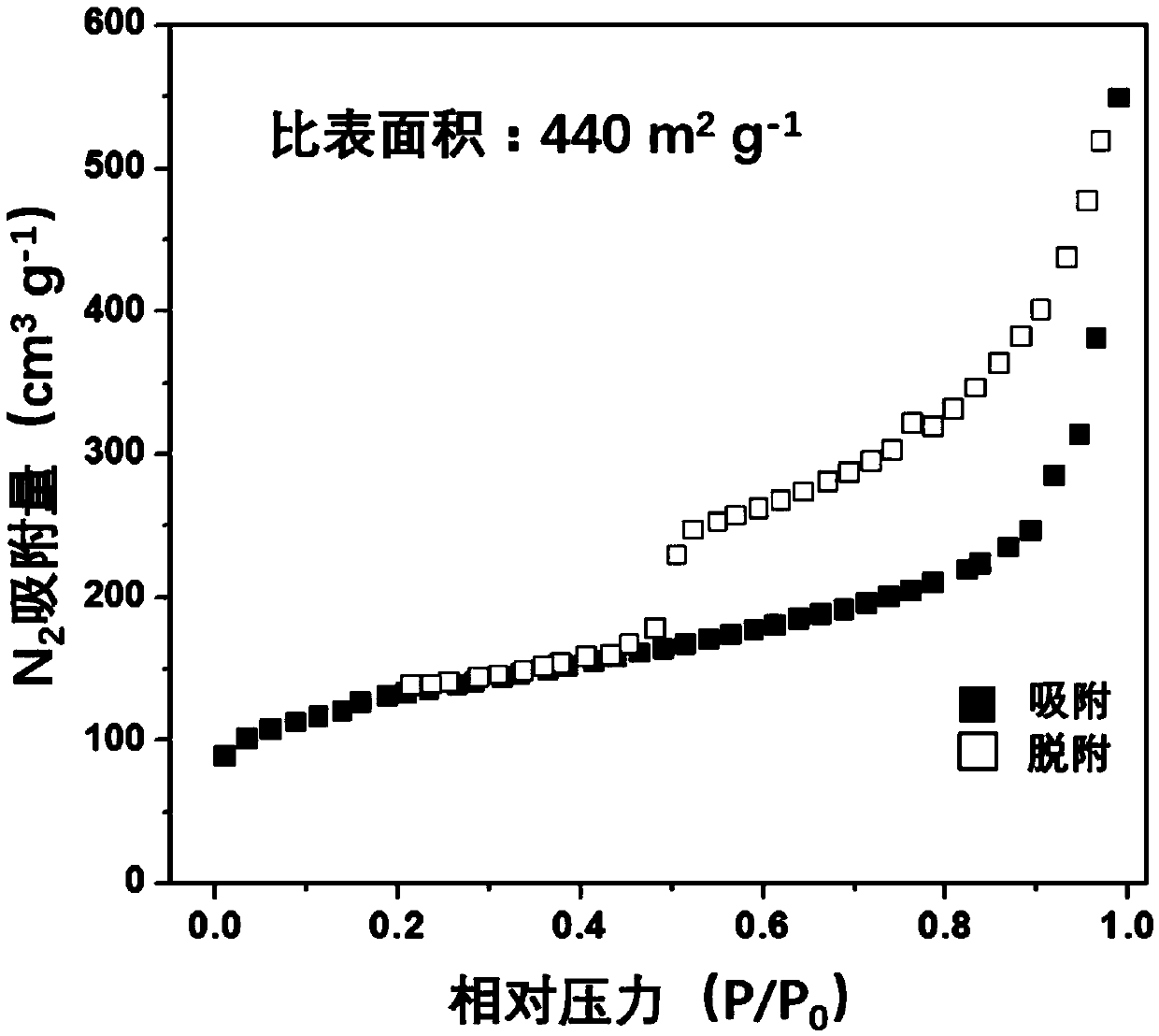
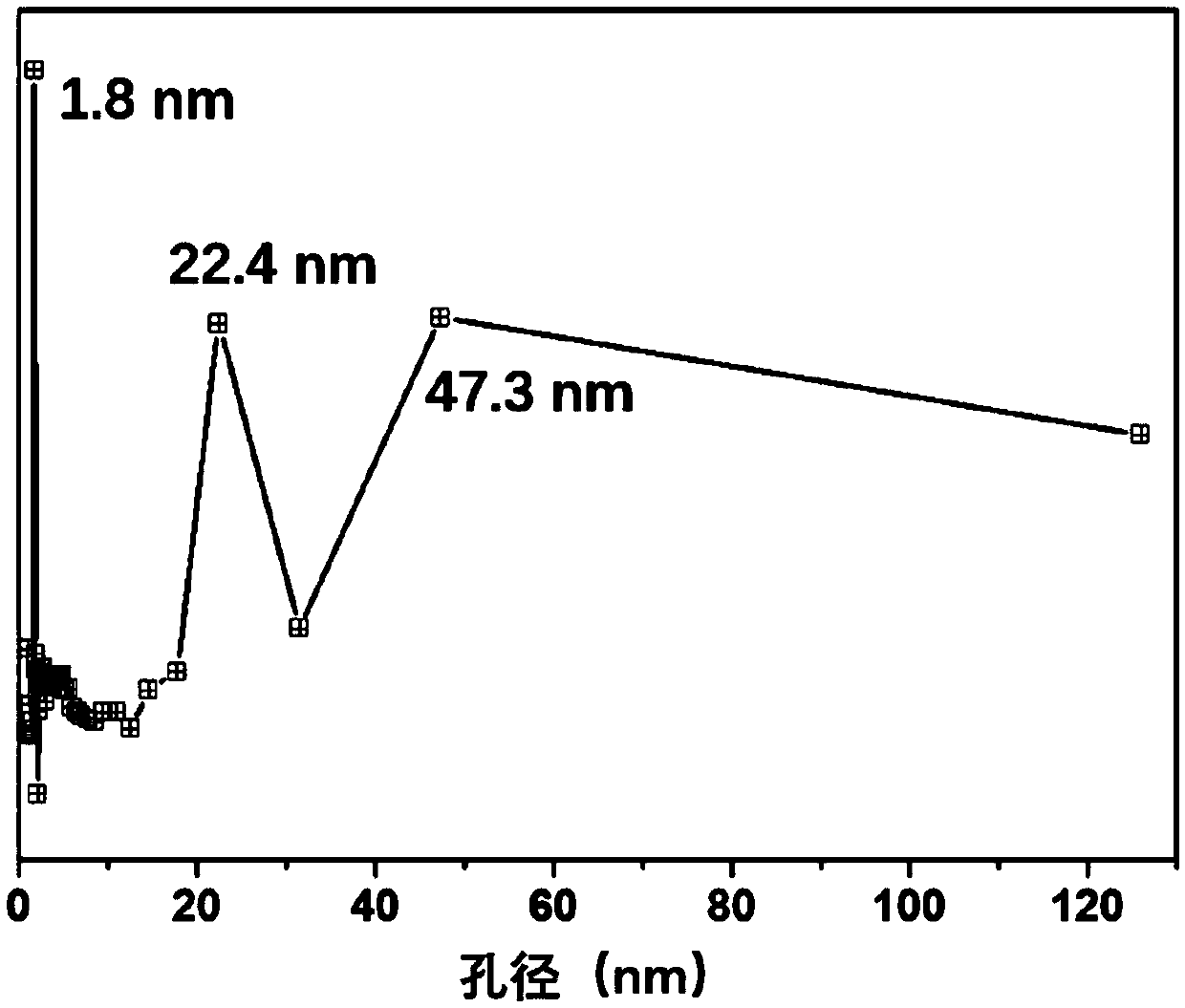
Preparation method of iron, cobalt and nitrogen co-doped hierarchical pore carbon nanosheet oxygen reduction catalyst
A technology of graded porous carbon and nanosheets, which is applied in electrical components, battery electrodes, circuits, etc., to achieve the effect of simple preparation method and high oxygen reduction activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Preparation of ZnO nanosheets
[0047] Add 55mL 0.02mol L to a 100mL Erlenmeyer flask –1 Zn(AC) 2 2H 2 O aqueous solution, under stirring, 5mL2mol L –1 Aqueous NaOH solution was added dropwise to the above solution, and after the dropwise addition was completed, it was stirred for 30 min, and a white precipitate was formed at room temperature. The mixture was then transferred to an autoclave and maintained at 150 °C for 3 h in an oven. After cooling to room temperature, ZnO nanosheets were obtained by centrifugation, washing, drying and crystallization.
[0048] (2) Preparation of core-shell structure ZnO@Zn / Fe / Co-ZIF precursor
[0049] Take step 1) 80.0 mg of ZnO nanosheets was added to an Erlenmeyer flask filled with a mixed solvent of DMF and water (64 mL, volume ratio 3:1), ultrasonicated for 20 min at room temperature to fully diffuse, and then 0.66 g of 2-methyl For imidazole, after ultrasonication for 5 minutes, add 20 mg of iron triacetylacetonate for...
Embodiment 2
[0055] (1) Preparation of ZnO nanosheets
[0056] Add 55mL 0.02mol L to a 100mL Erlenmeyer flask –1 Zn(AC) 2 2H 2 O aqueous solution, under stirring, 5mL2mol L –1 Aqueous NaOH solution was added dropwise to the above solution, and after the dropwise addition was completed, it was stirred for 30 min, and a white precipitate was formed at room temperature. The mixture was then transferred to an autoclave and maintained at 150 °C for 3 h in an oven. After cooling to room temperature, ZnO nanosheets were obtained by centrifugation, washing, drying and crystallization.
[0057] (2) Preparation of core-shell structure ZnO@Zn / Fe-ZIF precursor
[0058] Take step 1) 80.0 mg of ZnO nanosheets was added to an Erlenmeyer flask filled with a mixed solvent of DMF and water (64 mL, volume ratio 3:1), ultrasonicated for 20 min at room temperature to fully diffuse, and then 0.66 g of 2-methyl For imidazole, after ultrasonication for 5 minutes, add 20.0 mg of iron triacetylacetonate, mix...
Embodiment 3
[0064] (1) Preparation of ZnO nanosheets
[0065] Add 55mL 0.02mol L to a 100mL Erlenmeyer flask –1 Zn(AC) 2 2H 2 O aqueous solution, under stirring, 5mL2mol L –1 Aqueous NaOH solution was added dropwise to the above solution, and after the dropwise addition was completed, it was stirred for 30 min, and a white precipitate was formed at room temperature. The mixture was then transferred to an autoclave and maintained at 150 °C for 3 h in an oven. After cooling to room temperature, ZnO nanosheets were obtained by centrifugation, washing, drying and crystallization.
[0066] (2) Preparation of core-shell structure ZnO@Zn / Co-ZIF precursor
[0067] Take step 1) 80.0 mg of ZnO nanosheets was added to an Erlenmeyer flask filled with a mixed solvent of DMF and water (64 mL, volume ratio 3:1), ultrasonicated for 20 min at room temperature to fully diffuse, and then 0.66 g of 2-methyl Imidazole, after ultrasonication for 5 minutes, add 25.0 mg of cobalt nitrate hexahydrate, mix ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com