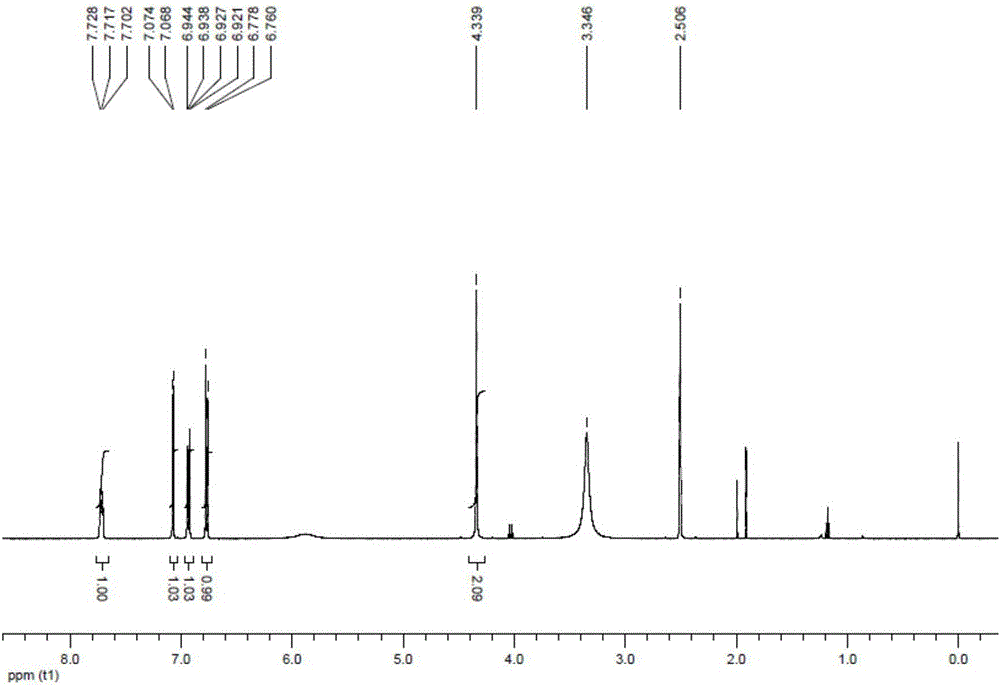
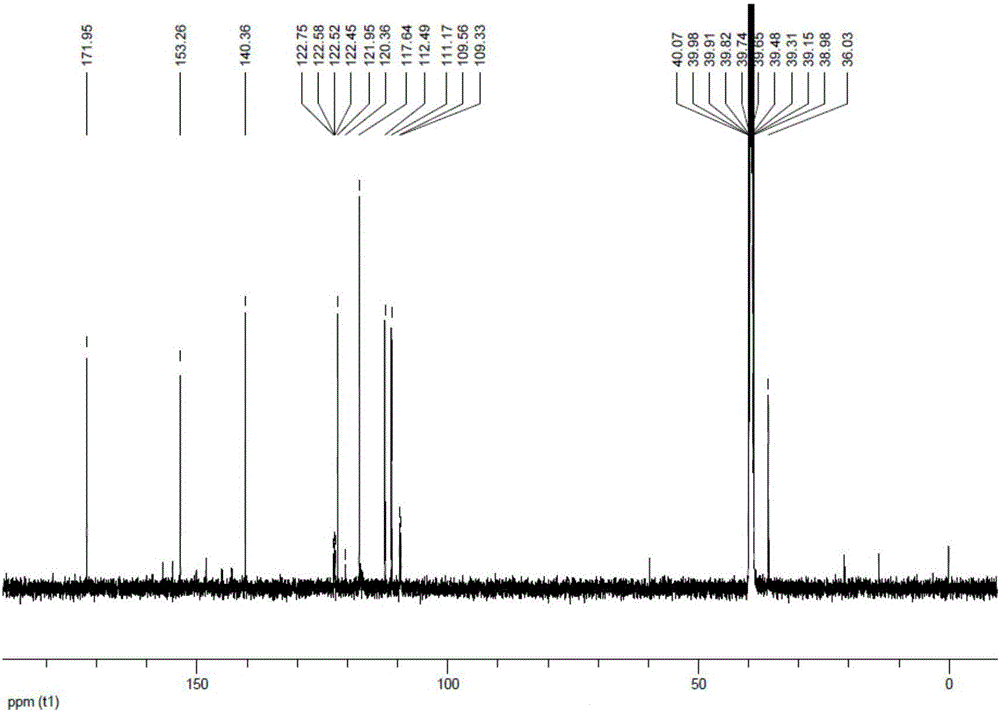
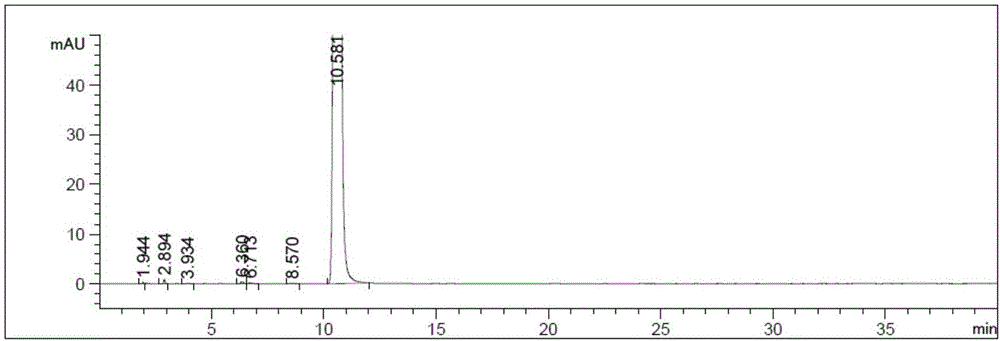
Salfaprodil impurity B, and preparation method and application thereof
A technology of impurity and reaction, applied in the field of sofadil impurity B and its preparation, can solve the problems of reducing drug efficacy, increasing requirements, toxic and side effects, etc., to improve the quality of finished products, strengthen control, improve accurate positioning and qualitative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The synthesis of embodiment 1 compound (Ⅲ)
[0043] Dissolve 42.13g of compound (II) in 210mL of water, lower the temperature to 0-10°C, add hydrochloric acid thereto, adjust the pH to 2-4, and stir at room temperature for half an hour. Extracted three times with ethyl acetate, combined the organic phases, washed once with saturated brine, dried over anhydrous sodium sulfate; filtered to remove sodium sulfate, and concentrated the filtrate under reduced pressure to obtain 38.1 g of compound (Ⅲ), yield 99%, HPLC purity 100% . The reaction formula is as follows:
[0044]
Embodiment 2
[0045] The synthesis of embodiment 2 compound (Ⅲ)
[0046] Dissolve 42.13g of compound (II) in 210mL of water, lower the temperature to 0-10°C, add hydrochloric acid thereto, adjust the pH to 2-4, and stir at room temperature for half an hour. Extracted three times with isopropyl acetate, combined the organic phases, washed once with saturated brine, dried over anhydrous sodium sulfate; filtered to remove sodium sulfate, and concentrated the filtrate under reduced pressure to obtain 37.2 g of compound (Ⅲ), yield 97%, HPLC purity 100 %.
Embodiment 3
[0047] The synthesis of embodiment 3 compound (I)
[0048] 38.1g of the compound (Ⅲ) prepared in Example 1 was dissolved in 250mL of methanol, and 3.8g of 5% palladium carbon and 13.8g of anhydrous potassium carbonate were added thereto; the reaction kettle was first replaced with nitrogen three times, and then replaced with hydrogen After three times, control the pressure to 1.0-2.0Mpa and react at a temperature of 40-50°C. After reacting for 8 hours, samples were taken for HPLC detection, and the reaction was stopped if the raw material content was less than or equal to 5%. The reaction solution was filtered to remove palladium carbon, and the filtrate was concentrated under reduced pressure to remove methanol. Add 100 mL of water to the concentrate, and adjust the pH to 2-4 with hydrochloric acid. Extracted three times with ethyl acetate, combined the organic phases, washed once with saturated brine, and dried over anhydrous sodium sulfate; the sodium sulfate was removed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com