Compound as well as preparation method, preparation and application thereof
A technology of compound and target compound, which is applied in the field of compound and its preparation, achieves less environmental pollution, simple and easy preparation method, and obvious anticoagulant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method of the compound described in the present invention is to take 2-chloro-4 hydroxybenzaldehyde or 2-bromo-4 hydroxybenzaldehyde as raw material and tetraacetylglucose trichloroacetimidate generation Schmidt reaction, then with NaBH4 or DAST The reaction is carried out to obtain the target compound.
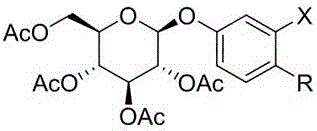
[0029] The reaction formula for this reaction is:
[0030] In an embodiment, the reaction is specifically 2-chloro-4-hydroxybenzaldehyde, 2-bromo-4-hydroxybenzaldehyde etc. and tetraacetylglucose trichloroacetimide ester under the condition of organic solvent and catalyst Schmidt reaction and then with NaBH 4, DAST, etc. to react to obtain the compound.
[0031] Organic solvents refer to a class of organic compounds that contain carbon atoms and can dissolve water-insoluble substances, including alkanes, alkenes, alcohols, aldehydes, amines, esters, ethers, ketones, aromatic hydrocarbons, hydrogenated hydrocarbons, terpene hydrocarbons, Halogenated h...
Embodiment 1
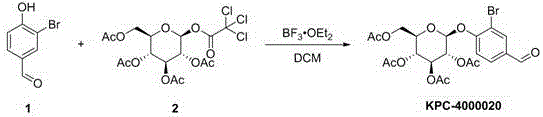
[0045] Preparation of KPC-4000020:
[0046]
[0047] Add compound 1 (400 mg, 1.99 mmol, 1.0 eq), compound 2 (1.76 g, 3.58 mmol, 1.8 eq) and molecular sieves (3.0 g, 4A) in a 25 ml two-necked flask. Replace nitrogen. Dichloromethane (5 mL) was successively added to the flask, and the flask was placed in an ice bath and stirred for 30 minutes. Add boron trifluoride diethyl ether (0.28 mL, 2.19 mmol, 1.1 eq) slowly dropwise with a syringe. Stirring was continued for 30 minutes after the dropwise addition was complete. Then the ice bath was removed, and the reaction solution was naturally warmed to room temperature (20°C). The reaction proceeded 8 times by TLC until the starting material disappeared (2 hours). Slowly pour the reaction solution into a beaker filled with crushed ice (20 g) to quench. After the ice was completely melted, the aqueous phase of the resulting mixture was extracted with ethyl acetate (20 mL × 3). The organic phases were combined and dried over an...
Embodiment 2
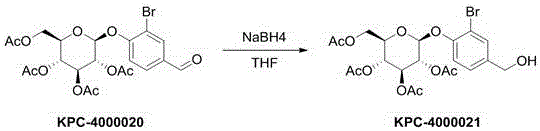
[0050] Preparation of KPC-4000021:
[0051]
[0052] Compound KPC-4000020 (300 mg, 0.56 mmol, 1.0 eq) was dissolved in 2.0 mL of methanol, cooled to 0 °C under nitrogen protection, and sodium borohydride (21 mg, 0.56 mmol, 1.0 eq) was added in portions. Then the ice bath was removed, and the reaction solution was naturally warmed to room temperature (20°C). The progress of the reaction was monitored by TLC until the starting material disappeared (1 hour). The reaction solution was slowly poured into a saturated solution of NH4Cl (20 mL). The aqueous phase of the resulting mixture was extracted with ethyl acetate (10 mL × 3). The organic phases were combined and dried over anhydrous sodium sulfate. The mixture was filtered. The solvent was removed on a rotary evaporator. The crude product was purified with a silica gel column. Eluent ratio: ethyl acetate / petroleum ether = 60%. Obtained white solid 221 mg, yield: 73%.
[0053] 1H NMR (400 MHz, CDCl3) δ 7.57 (s, 1H), 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com