Linoleate isomerase mutant and application thereof
A technology of linoleic acid isomerase and mutants, applied in the direction of isomerase, cis-trans isomerase, application, etc., can solve the problems of unsatisfactory enzyme activity and industrial application, and achieve good Industrial application prospects, the effect of improving enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
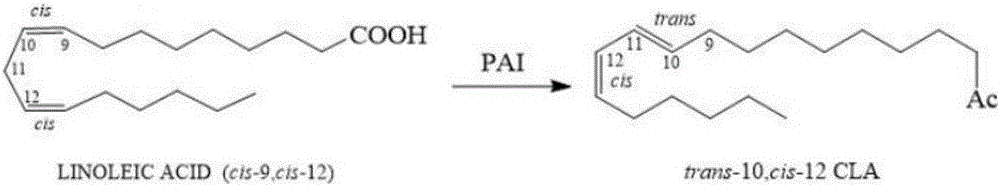
[0031] Wild-type linoleic acid isomerase (PAI; Gene Bank No. AX062088), but the enzyme activity of the wild-type linoleic acid isomerase is low, which cannot well meet the requirements of industrial applications.
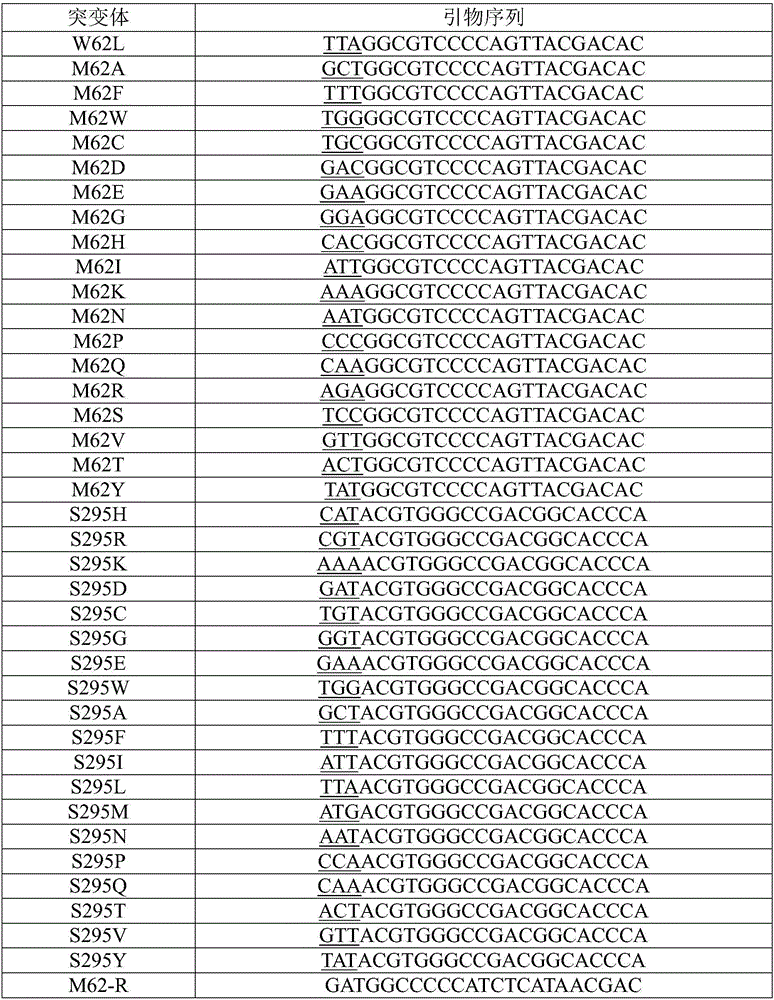
[0032] The wild-type linoleic acid isomerase amino acid sequence is shown in SEQ ID NO: 1, and the PET28a recombinant plasmid containing the wild-type linoleic acid isomerase gene (nucleotide sequence shown in SEQ ID NO: 2) ( With a histidine tag) as a template, saturation mutation was carried out at the M62 and S295 sites. Therefore, use oligo7 software to design blunt-end primers, and then perform PCR site-directed mutagenesis. The primers are shown in Table 1, wherein M62-R and S295-R are reverse primers, and the rest are forward primers. The primers are the same, and the first three bases in the forward primer are mutation sites. In this PCR mutation, KOD-Plus-Neo DNA polymerase kit (purchased from Toyobo Technology Co., Ltd., Shanghai) was used.
[0033] Tabl...
Embodiment 2
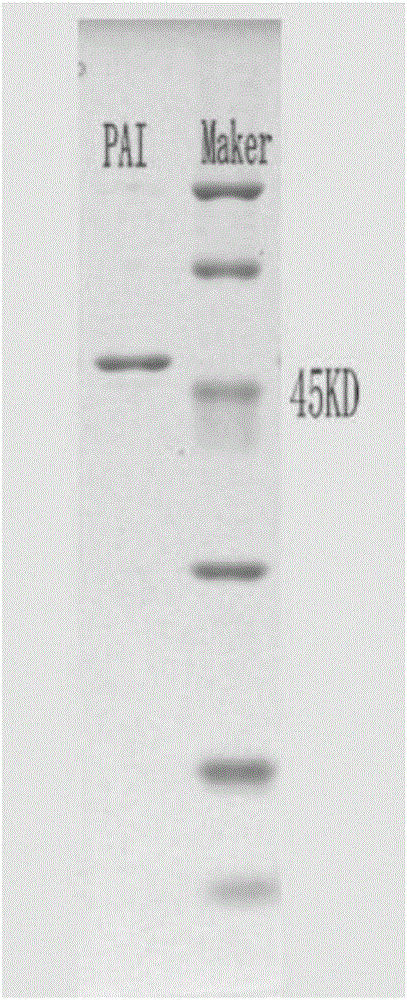
[0047] 1. Expression of linoleic acid isomerase and its mutants
[0048] (1) Transform the plasmid containing the wild-type linoleic acid isomerase gene and the mutant plasmid obtained in Example 1 into Escherichia coli JM109 (DE3) competent cells, and then spread it on an LB solid plate and incubate at 37°C for 10 h Afterwards, the growth status of the bacteria was observed, and the recombinant transformants were picked and inoculated in LB medium, and cultured at 37° C. for 18 hours to obtain recombinant transformants.
[0049] (2) Inoculate the recombinant transformant in LB liquid medium containing 5ml of kanamycin (50μg / ml), place it in a shaker at 37°C and 200rpm, and culture it until the OD600 reaches about 0.4-0.5, and obtain seed liquid.
[0050] (3) The seed liquid was inoculated in the self-inducing medium with an inoculation amount of 1% by volume, and cultured at 25° C. for 48 hours. The bacterial solution was broken up by ultrasonic waves until no cells were de...
Embodiment 3
[0057] Example 3 Detection of Enzyme Activity of Linoleic Acid Isomerase and Mutants thereof
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com