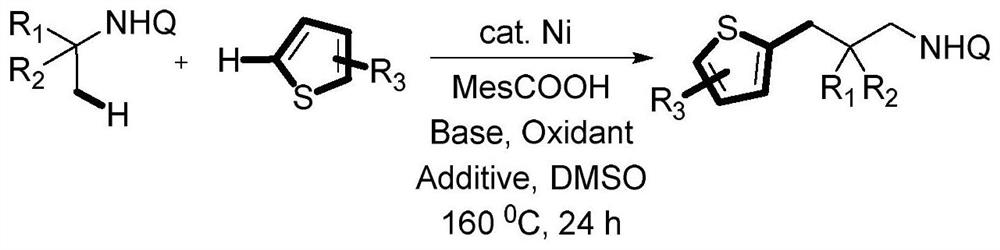
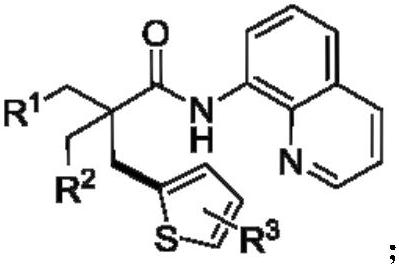
A Novel Catalytic Direct Dehydrogenation Coupling Method for the Synthesis of Thiophene-Containing Alkanes
A technology for dehydrogenation coupling and compounds, applied in the direction of organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0026] Add N-8-quinoline pivalamide (0.2mmol), nickel bromide (0.04mmol), 2,4,6-trimethylbenzoic acid (0.08mmol), potassium dihydrogen phosphate (0.4 mmol), thiophene (0.6mmol), silver carbonate (0.6mmol), tetrabutylammonium bromide (0.6mmol), DMSO (0.5ml) was added under nitrogen, and reacted at 160°C for 24h. After the reaction was completed, it was lowered to room temperature, 20ml of distilled water was added, extracted with ethyl acetate (3x10ml), dried by adding anhydrous sodium sulfate, and the product was separated by column chromatography with a yield of 64%.
preparation example 2
[0028] Add N-8-quinoline pivalamide (0.2mmol), nickel bromide (0.04mmol), 2,4,6-trimethylbenzoic acid (0.08mmol), potassium dihydrogen phosphate (0.4 mmol), 2-chlorothiophene (0.6mmol), silver carbonate (0.6mmol), tetrabutylammonium iodide (0.6mmol), DMF (0.5ml) was added under nitrogen, and reacted at 160°C for 24h. After the reaction was completed, it was lowered to room temperature, 20ml of distilled water was added, extracted with ethyl acetate (3 x 10ml), dried by adding anhydrous sodium sulfate, and the product was separated by column chromatography with a yield of 63%.
preparation example 3
[0030] Add N-8-quinoline pivalamide (0.2mmol), nickel bromide (0.04mmol), 2,4,6-trimethylbenzoic acid (0.08mmol), potassium dihydrogen phosphate (0.4 mmol), 2-bromothiophene (0.6mmol), silver carbonate (0.6mmol), tetrabutylammonium iodide (0.6mmol), DMF (0.5ml) was added under nitrogen, and reacted at 160°C for 24h. After the reaction was completed, it was lowered to room temperature, 20ml of distilled water was added, extracted with ethyl acetate (3 x 10ml), dried by adding anhydrous sodium sulfate, and the product was separated by column chromatography with a yield of 61%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com