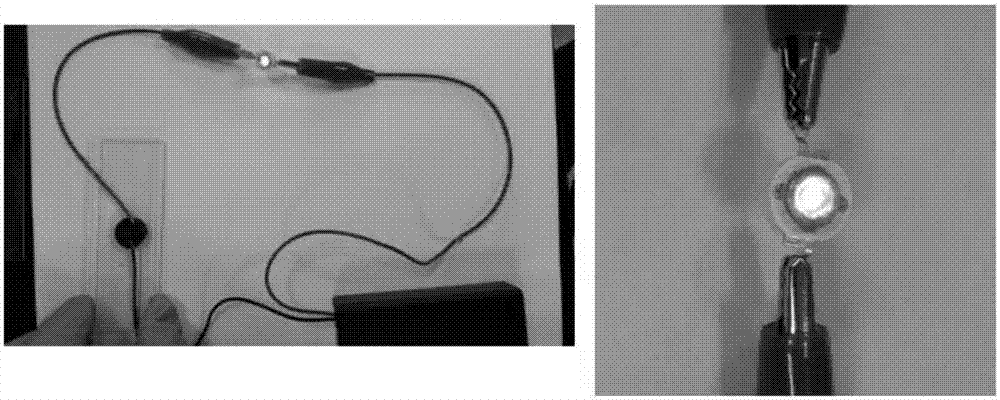
Preparation method of PNAGA/PANI self-repairing conductive hydrogel
A conductive hydrogel and self-repairing technology, which is applied in the preparation of PNAGA/PANI self-repairing conductive hydrogel and the field of self-repairing conductive hydrogel, can solve problems such as the impact of energy storage capacity, and achieve improved conductivity, The effect of excellent self-healing performance and excellent electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0022] Step 1: Add glycinamide hydrochloride (0.095mol) into a three-necked flask equipped with mechanical stirring and placed in an ice bath, blow nitrogen gas, add 10ml of ice water, and stir until completely dissolved. 40ml ice ether and 55ml ice 2mol L -1 K 2 CO 3 The solutions were successively added into the flask, 0.10 mol of acryloyl chloride was dissolved in 40 ml of ether, and added dropwise into a three-necked flask under stirring at 500 rpm, and then reacted at room temperature for 2 hours. Use 6M HCl solution to adjust the pH of the solution to 2. After removing the organic phase, the aqueous solution is washed three times with ether, the color of the solution is removed with charcoal, and filtered. The residual ether is removed by rotary evaporation at 20°C, and then the pH of the solution is adjusted with 2M NaOH solution. Adjust to neutrality, freeze-dry to obtain a crude product, wash three times with ethanol / methanol (4 / 1, v / v) solution, and obtain NAGA aft...
Embodiment example 2
[0026] Step 1: Add glycinamide hydrochloride (0.095mol) into a three-necked flask equipped with mechanical stirring and placed in an ice bath, blow nitrogen gas, add 10ml of ice water, and stir until completely dissolved. 40ml ice ether and 55ml ice 2mol L -1 K 2 CO 3 The solutions were successively added into the flask, 0.105mol of acryloyl chloride was dissolved in 40ml of ether, and added dropwise into a three-necked flask under stirring at 500rpm, and then reacted at room temperature for 2h. Use 6M HCl solution to adjust the pH of the solution to 2. After removing the organic phase, the aqueous solution is washed three times with ether, the color of the solution is removed with charcoal, and filtered. The residual ether is removed by rotary evaporation at 20°C, and then the pH of the solution is adjusted with 2M NaOH solution. Adjust to neutrality, freeze-dry to obtain a crude product, wash three times with ethanol / methanol (4 / 1, v / v) solution, and obtain NAGA after the ...
Embodiment example 3
[0030] Step 1: Add glycinamide hydrochloride (0.095mol) into a three-necked flask equipped with mechanical stirring and placed in an ice bath, blow nitrogen gas, add 10ml of ice water, and stir until completely dissolved. 40ml ice ether and 55ml ice 2mol L -1 K 2 CO 3The solutions were successively added into the flask, 0.10 mol of acryloyl chloride was dissolved in 40 ml of ether, and added dropwise into a three-necked flask under stirring at 500 rpm, and then reacted at room temperature for 2 hours. Use 6M HCl solution to adjust the pH of the solution to 2. After removing the organic phase, the aqueous solution is washed three times with ether, the color of the solution is removed with charcoal, filtered, and the residual ether is removed by rotary evaporation at 20°C, and then the pH of the solution is adjusted with 2M NaOH solution. Adjust to neutrality, freeze-dry to obtain a crude product, wash three times with ethanol / methanol (5 / 1, v / v) solution, and obtain NAGA afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com