Alkaline battery negative pole material and preparation method thereof
A negative electrode material, alkaline battery technology, applied to battery electrodes, circuits, electrical components, etc., can solve problems such as charging difficulties, poor discharge capacity, and increased resistance of zinc electrodes, and achieve high-rate charge-discharge performance and high charge-discharge performance Receptivity, the effect of improving the hydrogen evolution overpotential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
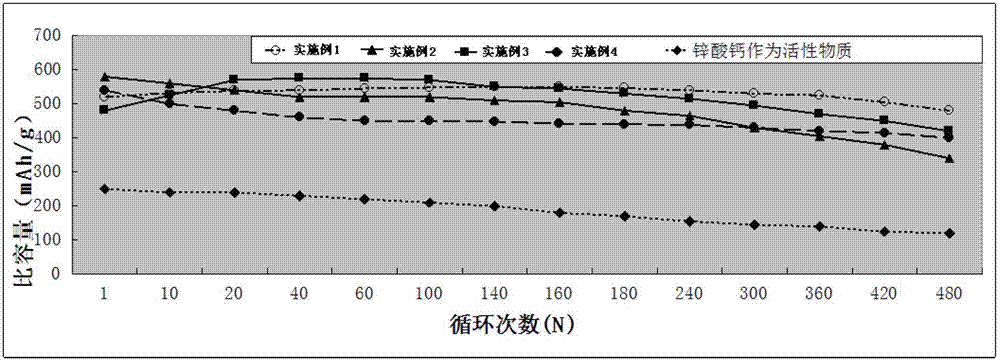
Image
Examples
Embodiment 1
[0038] Step 1: Dissolve 1 mol of zinc nitrate in 1 liter of deionized water, add 0.3 mol of gallium chloride, 0.1 mol of ferric nitrate, and 0.02 mol of lanthanum sulfate, fully dissolve, and prepare a solution with a zinc ion concentration of 1 mol / L. Polyethylene glycol was added in the solution as a dispersant in an amount of 8 grams per liter.
[0039] Step 2: Take 2.5 mol potassium hydroxide and 0.2 mol anhydrous sodium carbonate and fully dissolve them with deionized water to prepare a 2 mol / L potassium hydroxide solution and use it as a precipitant.
[0040] Step 3: Slowly add the precipitating agent dropwise to the zinc sulfate solution under the stirring of the agitator, and control the temperature at 35°C. When the pH value of the reaction system reaches 9.5, the dropping of the precipitating agent is completed and the aging process continues for 7 hours, filtered to obtain a precipitate, then repeatedly washed the precipitate until the filtrate was neutral, and drie...
Embodiment 2
[0044] Step 1: Dissolve 1 mol of zinc sulfate in 2 liters of deionized water, add 0.1 mol of ferric sulfate, 0.002 mol of gallium sulfate, and 0.005 mol of cerium nitrate, fully dissolve, and prepare a solution with a zinc ion concentration of 0.5 mol / L. Add polyvinylpyrrolidone to it in an amount of 8 grams per liter.
[0045] Step 2: Take 2.5 mol potassium hydroxide and 0.01 mol of sodium carbonate to fully dissolve with deionized water, and configure it into a 4 mol / L potassium hydroxide solution, which is used as a precipitant.
[0046] Step 3: Slowly add the precipitating agent dropwise to the zinc sulfate solution while the agitator is stirring, and control the temperature at 30°C. When the pH value of the reaction system reaches 10, the precipitating agent is added dropwise and continue to age for 8 hours, filtered to obtain a precipitate, and then repeatedly washed the precipitate until the filtrate was neutral, and dried to constant weight.
[0047] Step 4: Put the d...
Embodiment 3
[0050] Step 1: Dissolve 1.5 mol of zinc nitrate in 2 liters of deionized water, add 0.05 mol of chromium nitrate, 0.1 mol of cobalt nitrate, and 0.01 mol of yttrium chloride, fully dissolve, and prepare a solution with a zinc ion concentration of 0.75 mol / L. To this solution was added polyethylene glycol as a dispersant in an amount of 10 g / liter.
[0051] Step 2: Take 3.4mol potassium hydroxide and 0.02mol sodium carbonate and fully dissolve them with deionized water to prepare a 4mol / L potassium hydroxide solution and use it as a precipitant.
[0052] Step 3: Slowly add the precipitating agent dropwise to the zinc sulfate solution while stirring with the agitator, and control the temperature at 35°C. When the pH value of the reaction system reaches 9, the precipitating agent is added dropwise and continue to age for 10 hours, filtered to obtain a precipitate, then repeatedly washed the precipitate until the filtrate was neutral, and dried to constant weight
[0053] Step 4:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com