Mesenchymal stem cell derived cartilage cell preparation and preparation method thereof
A technology of chondrocytes and mesenchymal stem cells, applied in skin care preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problem of low survival rate and value-added rate of chondrocytes, increased pain and scars of patients, and transplantation Poor effect and other problems, to achieve the effect of shortening the operation cycle, improving the probability of success, and low discharge rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 of the present invention provides a chondrocyte preparation derived from mesenchymal stem cells, including a first injection. The first injection is mainly composed of raw materials, auxiliary material I, and chondrocytes configured with water for injection. Contain described raw material 0.25mg, described adjuvant 10.5mg, described chondrocyte 1 * 10 in water 5 , the raw material is mainly made of the following ingredients in grams by weight:
[0034] Zushi Hemp Extract 0.075mg
[0035] Danshen Extract 0.0875mg
[0036] Dog Ridge Extract 0.0875mg.
Embodiment 2
[0038] Embodiment 2 of the present invention provides a chondrocyte preparation derived from mesenchymal stem cells, including a first injection, the first injection is mainly composed of raw materials, auxiliary material I, and chondrocytes configured with water for injection, and each mL of the injection Water contains 1.25 mg of the raw material, 1.5 mg of the auxiliary material I, and 1 × 10 chondrocytes 8 , the raw material is mainly made of the following ingredients in grams by weight:
[0039] Zushi Hemp Extract 0.125mg
[0040] Danshen Extract 0.625mg
[0041] Dog Ridge Extract 0.500mg.
[0042] A preparation method of a chondrocyte preparation derived from mesenchymal stem cells, the preparation method comprising the following steps:
[0043] S1. Chondrocytes induced and differentiated using adipose-derived mesenchymal stem cells;
[0044] S2. Preparation of raw materials: Weigh 0.125 mg of Zushima extract, 0.625 mg of Salvia miltiorrhiza extract, and 0.500 mg of ...
Embodiment 3
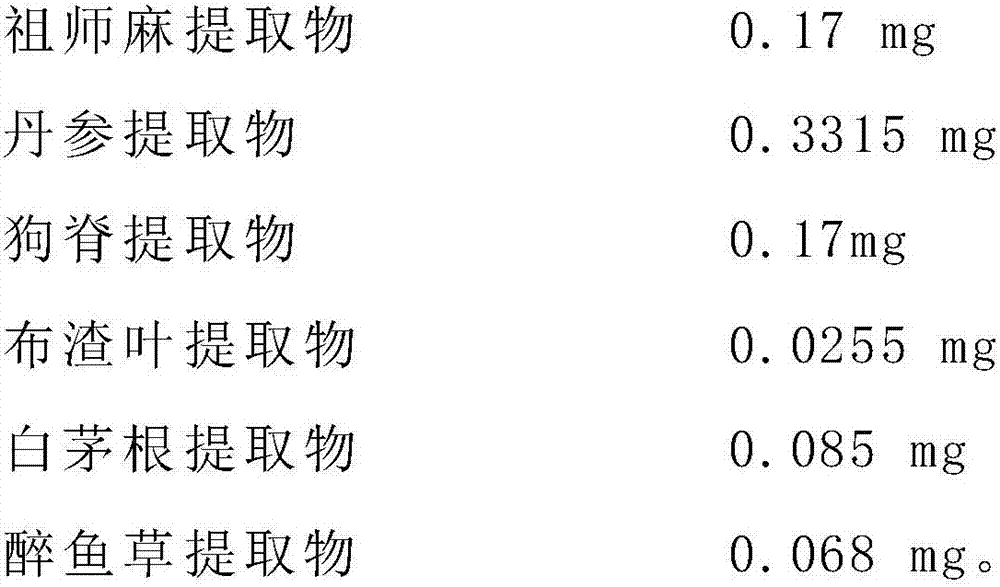
[0052] Embodiment 3 of the present invention provides a chondrocyte preparation derived from mesenchymal stem cells, including a first injection. The first injection is mainly composed of raw materials, auxiliary material I, and chondrocytes configured with water for injection. The water contains 0.85 mg of the raw material, 1.0 mg of the auxiliary material I, and 1 × 10 chondrocytes. 7 , the raw material is mainly made of the following ingredients in grams by weight:
[0053]
[0054] The preparation method of this preparation is identical with embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com