Method for preparing trans-1,3,3,3-tetrafluoropropene
A technology for tetrafluoropropene and pentafluoropropane is applied in the field of preparing trans-1,3,3,3-tetrafluoropropene, which can solve the problems of difficult recovery and achieve the effect of high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The reaction was carried out in a stirred 500mL stainless steel autoclave. 200g of 50% aqueous KOH solution, 200g of chlorobenzene, 1.2g of polyethylene glycol 2000 and 119g of HFC-245fa were successively dropped into the reactor, and the stirring was started, and the reaction temperature was raised to 70°C. After 60 minutes of reaction, the reaction temperature was maintained, from The gas phase port of the reactor slowly exhausted the reaction materials with low boiling points, collected them in cold hydrazine at -30°C, and analyzed the collected materials by gas chromatography. The results showed that the conversion rate of HFC-245fa was 95.9%, and the total selection of HFO-1234ze The specificity is 99.5%, and the selectivity of E-HFO-1234ze is 78.4%.
Embodiment 2
[0021] Example two is similar to example one, except that the aromatic hydrocarbon solvent chlorobenzene is not added, the reaction results show that the conversion rate of HFC-245fa is 20.1%, and the total selectivity of HFO-1234ze is 99.3%, wherein E-HFO-1234ze The selectivity is 75.2%. The results of Comparative Example 1 show that the dehydrofluorination activity of the alkaline aqueous solution is greatly improved after the addition of chlorobenzene.
Embodiment 3
[0023] The difference from Example 1 is that the chlorobenzene solvent in this example is the recovery solvent of Example 1, specifically: after the reaction solution in the kettle in Example 1 is lowered to room temperature, it is placed in a stratifier for phase separation, and the lower layer is removed. The organic phase is the chlorobenzene solvent. The recovered chlorobenzene is directly used in the reaction of this embodiment, and other operations remain unchanged. The reaction results show that the conversion rate of HFC-245fa is 95.8%, and the total selectivity of HFO-1234ze is 99.4%. The selection of E-HFO-1234ze The specificity is 79.1%, and the analysis result shows that the solvent can be recycled directly.
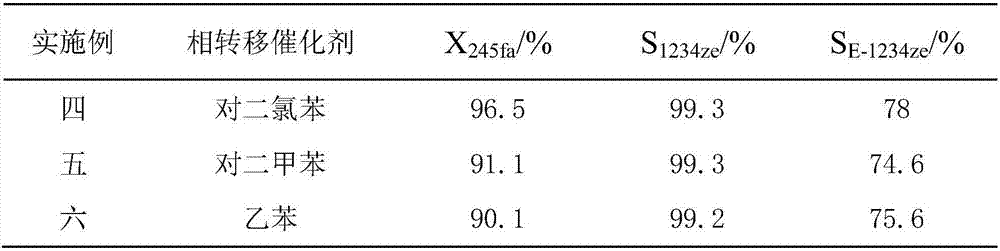
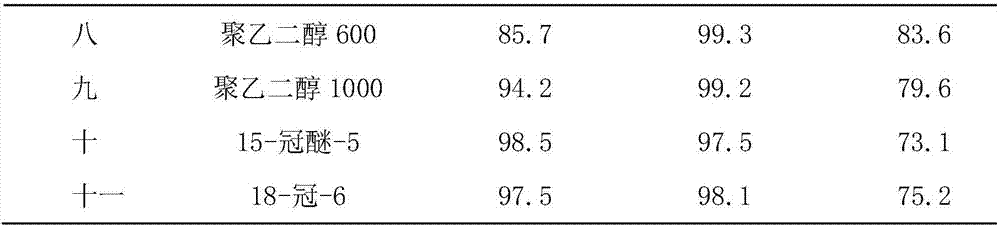
[0024] Embodiment four to six
[0025] The operation process of Examples 4-6 is similar to that of Example 1, the difference is that the solvent is changed, and the reaction results are shown in Table 1.
[0026] Table 1
[0027]
[0028] Note: X 245fa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com