Environment-friendly sulfur-containing gold leaching reagent and preparation method thereof
A gold leaching agent and environmental protection technology, which is applied in the field of sulfur-containing environmental protection gold leaching agent and its preparation, can solve the problems of thiourea oxidative decomposition, thiourea dosage and high cost, and achieve the effect of operating environment friendly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
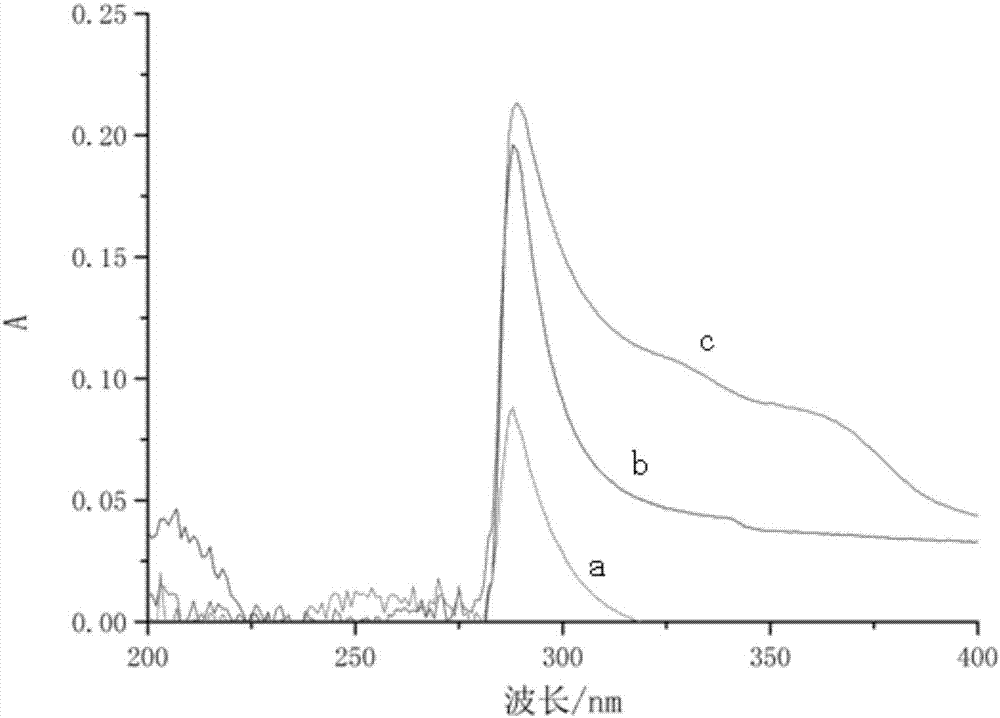
[0021] Embodiment 1: Get 8g of sodium thiosulfate pentahydrate and 4g of sodium tetrasulfide, dissolve them in 100mL of water, put them in a reaction kettle, add 10mL of ammonia water, slowly add 20mL of 1wt% bromine water, the reaction temperature is 55°C, and the reaction process is sampled. Observe that the absorption peak of sodium thiosulfate at wavelength 217nm weakens at the UV spectrophotometer, and the product absorbs at 288nm, and the absorption peak at 217nm disappears substantially (at this time, the solution turns pale yellow), and the absorption spectrum is as follows: figure 1 Shown in a, stop reacting at this moment. Then add 2 mL of saturated zinc sulfate solution to precipitate excess sulfur and polysulfide; filter and collect the filtrate to obtain 110 mL of liquid gold leaching agent.
[0022] Get 10mL of liquid gold leaching agent, 0.12g of copper sulfate pentahydrate, 6mL of ammonia water containing 28% ammonia, add tap water to form a 300mL solution; get...
Embodiment 2
[0023] Embodiment 2: Sodium tetrasulfide crystal 10g, be dissolved in 100mL water, be placed in reaction kettle, add the ammoniacal liquor 20mL of content 28%, this moment solution pH value is 11, then slowly add the bleaching powder emulsion of 15wt%, at normal temperature After the reaction, when the solution turns light yellow, stop adding the oxidant, take a sample and observe it with a UV spectrophotometer and find that there is a maximum absorption peak at 288nm, and the absorption spectrum is as follows: figure 1 Shown in b. Then add 5 mL of saturated zinc sulfate ammonia complex solution to precipitate excess sulfur radicals and polysulfide radicals; filter, wash the filter residue, collect the filtrate, and obtain 160 mL of liquid gold leaching agent.
[0024] Take 50mL of liquid gold leaching agent, 0.4g of copper sulfate, 10mL of ammonia water with an ammonia content of 28%, and add tap water to form a 300mL solution; take 100g of fine-grained fine gold ore with a g...
Embodiment 3
[0025] Embodiment 3: Get sublimated sulfur 30g, calcium hydroxide 15g, be dissolved in 100mL water, be placed in reactor, reaction temperature 95 ℃, this moment solution pH value is 11, then slowly add the bleaching powder emulsion of 15wt% until The average chemical valence of sulfur in the feed liquid is 2~3 (solution becomes light yellow at this moment), stop adding oxidizing agent, sampling has maximum absorption peak at 288nm place of ultraviolet spectrophotometer observation, and absorption spectrum is as follows figure 1 Shown in c. Then add 5 mL of zinc sulfate ammonia complex solution to precipitate excess sulfur and polysulfide; filter, wash the filter residue, collect the filtrate, and obtain 200 mL of liquid gold leaching agent. The liquid gold leaching agent was evaporated and crystallized to obtain 17.8 g of solid gold leaching agent.
[0026] Take 0.3g of solid gold leaching agent, 0.24g of copper sulfate pentahydrate, 6mL of ammonia water containing 28% ammoni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com