Method for producing high-concentration ferrate through step-by-step electrolytic process
A ferrate, high concentration technology, applied in the field of electrochemistry, can solve the problems of low ferrate concentration, low current efficiency, easy passivation of electrodes, etc., and achieve high product concentration, high current efficiency, and high reaction efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
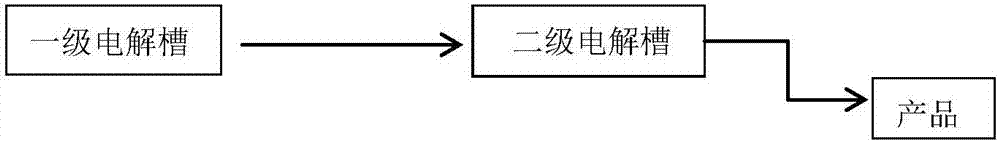
[0024] Example 1 The structure of the secondary electrolytic cell of the present invention
[0025] figure 1 A schematic diagram of the secondary electrolysis device of the present invention is given. Both electrolyzers have the same structure as the prior art electrolyzers. Perfluorinated cation exchange membranes are used to separate the cathode chamber and the anode chamber. Wire mesh or other iron materials are used as anodes, and nickel mesh or other metal materials are used as cathodes. , Direct current is applied between the anode and the cathode. The first-level electrolyzer uses sodium hydroxide as the electrolyte, and the second-level electrolyzer starts to use the sodium hydroxide and ferrate obtained by electrolysis in the first-level electrolyzer as the raw material anolyte, and still uses sodium hydroxide as the catholyte. Continue electrolysis. The raw material anolyte can be transported from the first-stage electrolytic cell to the second-stage electrolytic cell...
Embodiment 2
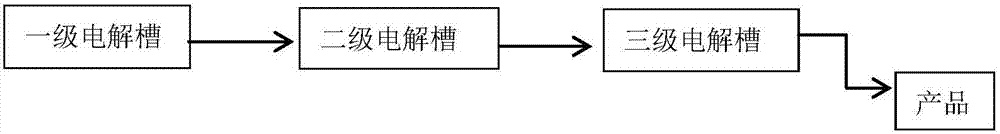
[0026] Example 2 The structure of the three-stage electrolytic cell of the present invention
[0027] figure 2 A schematic diagram of the three-stage electrolysis device of the present invention is given. The three electrolytic cells have the same structure as the electrolytic cell of the prior art, and the specific structure is the same as that of Embodiment 1. The first-level electrolyzer uses sodium hydroxide as the electrolyte, the second-level electrolyzer uses the sodium hydroxide and ferrate obtained by electrolysis in the first-level electrolyzer as raw material anolyte, and the third-level electrolyzer uses the second-level electrolysis The sodium hydroxide and ferrate obtained by electrolysis in the tank are used as the raw anolyte, and the three electrolytic tanks all use sodium hydroxide as the catholyte for electrolysis. The raw material anolyte obtained by the electrolysis of the first and second stage electrolyzers is transported by a pump, or circulated by heigh...
Embodiment 3
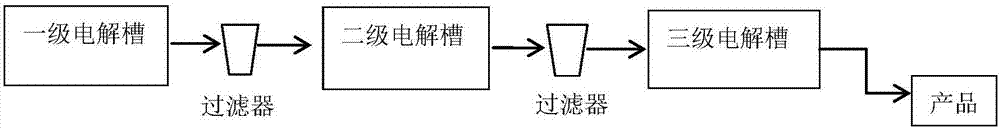
[0028] Example 3 Comparison between the production process of the present invention and the prior art
[0029] Using the same structure and size of the electrolysis cell and electrode, under the same current and bath temperature, under the same electrolyte volume flow, that is, the same output, using the production device process of the present invention, the product concentration is the original primary electrolysis The groove process is about twice or more. For example, a secondary or tertiary electrolysis process of the present invention (such as figure 1 , figure 2 ) The experimental results compared with the original first-level electrolysis process: the anode volume of the electrolytic cell is 150mL, and the current density is 40mA / cm at 48℃ 2 , The anolyte material flow rate is 2.5mL / min, that is, the residence time in the anode compartment of each electrolytic cell is 1 hour. With electrodes of the same material, structure and size, no matter the first, second or third st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com