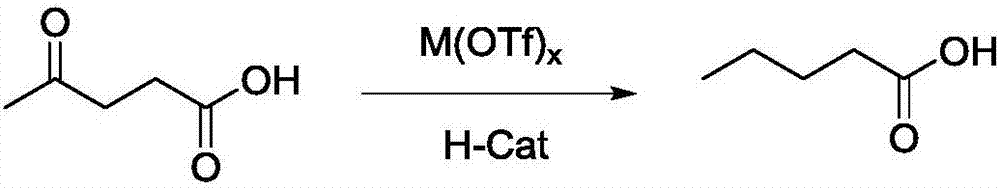
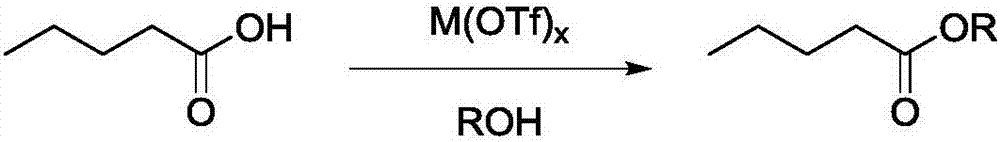
Method for preparing valeric acid and valerate from levulinic acid
A technology of levulinic acid and valeric acid ester, which is applied in the direction of carboxylate preparation, carboxylate preparation, chemical instruments and methods, etc., can solve the problems of environmental pollution, equipment corrosion, etc., achieve high utilization rate, mild reaction conditions, The effect of high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A method for preparing valeric acid from levulinic acid, comprising: adding 0.1mol levulinic acid to a reaction vessel, then adding 100ml of n-octane as a reaction solvent and 0.5mol% Pd / C based on levulinic acid and 2mol% Hf(OTf) 4 , after 3 replacements with hydrogen gas, the temperature was raised to 150°C under stirring, and the hydrogenolysis reaction was catalyzed for 6 hours under a hydrogen atmosphere with a pressure of 5 MPa. After cooling to room temperature, the reaction solution was obtained, and valeric acid was obtained by distillation under reduced pressure.
[0028] Sampling was diluted, and the yield of valeric acid determined by gas chromatography (GC) was 95%, and the purity was 99%. Among them, gas chromatography detection conditions: Shimadzu GC-2014C, DM-wax column, gasification temperature: 260 °C, detector temperature: 280 °C, column temperature: 100 °C (5min) → 10 °C / min → 180 °C (3min )→20°C / min→240°C (10min).
Embodiment 2
[0030] A method for preparing valeric acid from levulinic acid, comprising: adding 0.1mol levulinic acid to a reaction vessel, then adding 100ml of n-octane as a reaction solvent and 0.1mol% Pd / C based on levulinic acid and 10mol% Al(OTf) 3 , after 4 substitutions with hydrogen gas, the temperature was raised to 200°C under stirring, and the hydrogenolysis reaction was catalyzed for 10 h under a hydrogen atmosphere with a pressure of 10 MPa. After cooling to room temperature, the reaction solution was obtained, and valeric acid was obtained by distillation under reduced pressure.
[0031] Sampling was diluted, and the yield of valeric acid determined by gas chromatography (GC) was 92%, and the purity was 99%.
Embodiment 3
[0033] A method for preparing valeric acid from levulinic acid, comprising: adding 0.1mol levulinic acid to a reaction vessel, then adding 100ml of dioxane as a reaction solvent and 5mol% Ru / C based on levulinic acid and 0.1mol% Zr(OTf) 4 , after replacing twice with hydrogen gas, the temperature was raised to 100°C under stirring, and the catalytic hydrogenolysis reaction was carried out under a hydrogen atmosphere with a pressure of 8 MPa for 4 hours. After cooling to room temperature, the reaction liquid was obtained, and then valeric acid was obtained by distillation under reduced pressure.
[0034]Sampling was diluted, and the yield of valeric acid determined by gas chromatography (GC) was 94%, and the purity was 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com