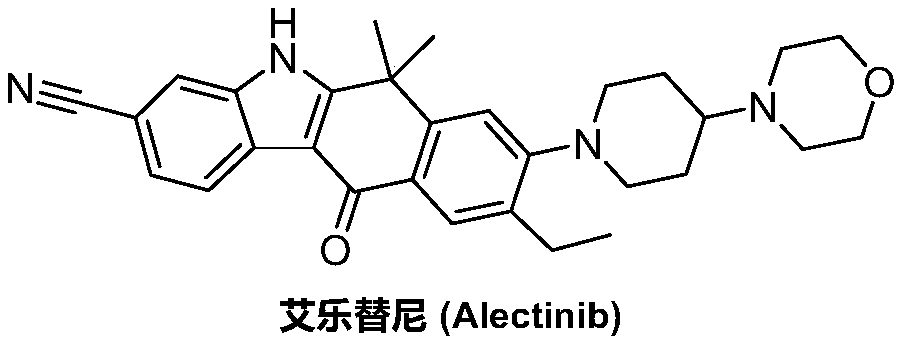
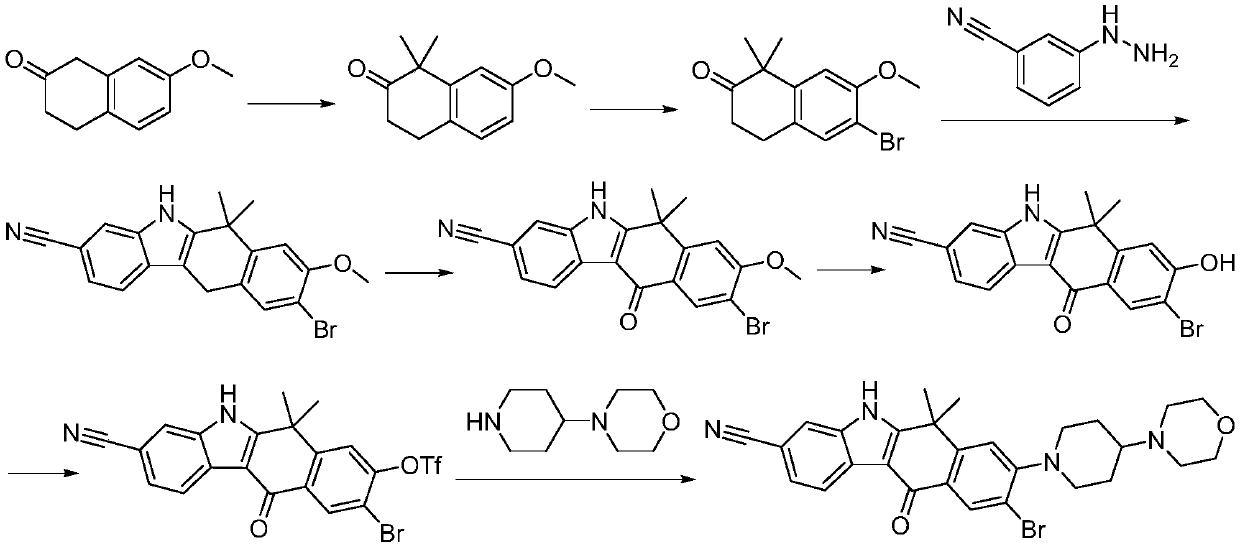
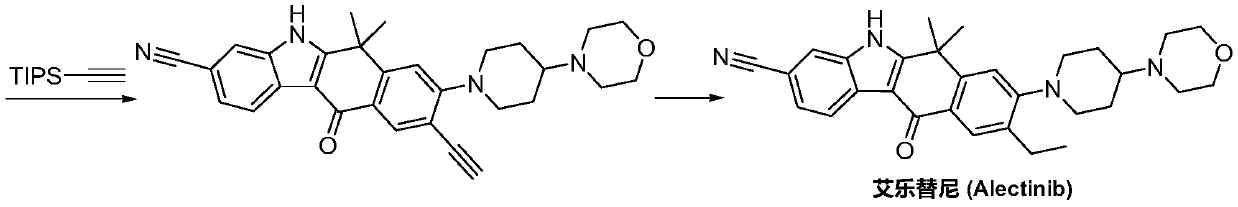
A kind of preparation method of alectinib
A technology of alectinib and ethyl, applied in the field of medicinal chemical synthesis, can solve the problems of unfavorable industrialized production and promotion, use a large amount of solvents, expensive starting materials, etc., and achieves easy and effective control of reaction conditions, easy availability of reagents, and impurities. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A) Preparation of 6-cyano-2-[2-(4-ethyl-3-iodophenyl)propan-2-yl]-1H-indole-3-carboxylic acid:
[0033] 4-(4-ethyl-3-iodophenyl)-4-methyl-3-oxopentanoic acid tert-butyl ester (6.3g, 15mmol), 3-cyanophenylhydrazine (2.5g, 19mmol) and three Fluoroacetic acid (94.9g, 832mmol) was mixed, the reaction mixture was stirred and reacted at 100°C for 9 hours, the reaction solution was lowered to 60°C, water (50mL) was added, and the reaction was kept for 3 hours, concentrated by rotary evaporation to dryness, and added to saturated sodium bicarbonate solution And, add ethyl acetate to extract, dry over magnesium sulfate, concentrate to dryness by rotary evaporation, recrystallize with ethyl acetate and n-hexane mixed solvent to get 6-cyano-2-[2-(4-ethyl-3-iodo Phenyl)propan-2-yl]-1H-indole-3-carboxylic acid, off-white solid (6.2g), yield 90%.
[0034] B) Preparation of 9-ethyl-6,6-dimethyl-8-iodo-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile:
[0035] 6-cyano-2-[2-(4-...
Embodiment 2
[0039] A) Preparation of 6-cyano-2-[2-(4-ethyl-3-iodophenyl)propan-2-yl]-1H-indole-3-carboxylic acid:
[0040]4-(4-Ethyl-3-iodophenyl)-4-methyl-3-oxopentanoic acid tert-butyl ester (11.0g, 26mmol), 3-cyanophenylhydrazine (4.8g, 36mmol) and acetic acid (103.1g, 1717mmol) were mixed, the reaction mixture was stirred and reacted at 115°C for 6 hours, the reaction solution was lowered to 70°C, water (110mL) was added, and the reaction was kept for 2 hours, concentrated by rotary evaporation to dryness, neutralized by adding saturated sodium bicarbonate solution, Add ethyl acetate for extraction, dry over magnesium sulfate, concentrate to dryness by rotary evaporation, and recrystallize with a mixed solvent of ethyl acetate and n-hexane to obtain 6-cyano-2-[2-(4-ethyl-3-iodophenyl )prop-2-yl]-1H-indole-3-carboxylic acid, off-white solid (9.6g), yield 81%.
[0041] B) Preparation of 9-ethyl-6,6-dimethyl-8-iodo-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile:
[0042] 6-cya...
Embodiment 3
[0046] A) Preparation of 6-cyano-2-[2-(4-ethyl-3-iodophenyl)propan-2-yl]-1H-indole-3-carboxylic acid:
[0047] 4-(4-Ethyl-3-iodophenyl)-4-methyl-3-oxopentanoic acid tert-butyl ester (3.6g, 9mmol), 3-cyanophenylhydrazine (1.4g, 11mmol) and formic acid (16.9g, 367mmol) were mixed, the reaction mixture was stirred and reacted at 90°C for 12 hours, the reaction solution was lowered to 50°C, water (45mL) was added, and the reaction was kept for 4 hours, concentrated by rotary evaporation to dryness, neutralized by adding saturated sodium bicarbonate solution, Add ethyl acetate for extraction, dry over magnesium sulfate, concentrate to dryness by rotary evaporation, and recrystallize with a mixed solvent of ethyl acetate and n-hexane to obtain 6-cyano-2-[2-(4-ethyl-3-iodophenyl )prop-2-yl]-1H-indole-3-carboxylic acid, off-white solid (3.3g), yield 80%.
[0048] B) Preparation of 9-ethyl-6,6-dimethyl-8-iodo-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile:
[0049] 6-cyano-2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com