Spirosolane glycoalkaloid, and preparation method and use thereof
A technology of spirosterane and alkaloids, which is applied in the field of preparation of glycoalkaloids, can solve the problems that the efficacy and interaction of components cannot be fully confirmed, complex components, etc., and achieve the effect of definite function and single component
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
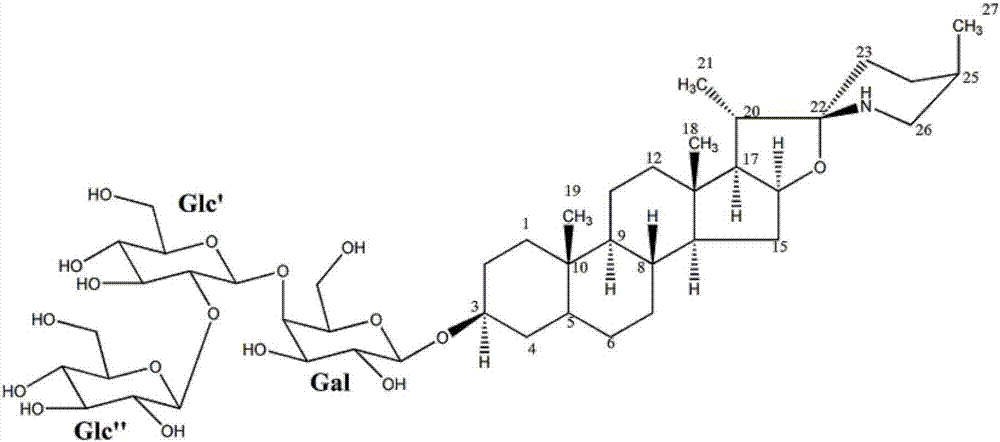
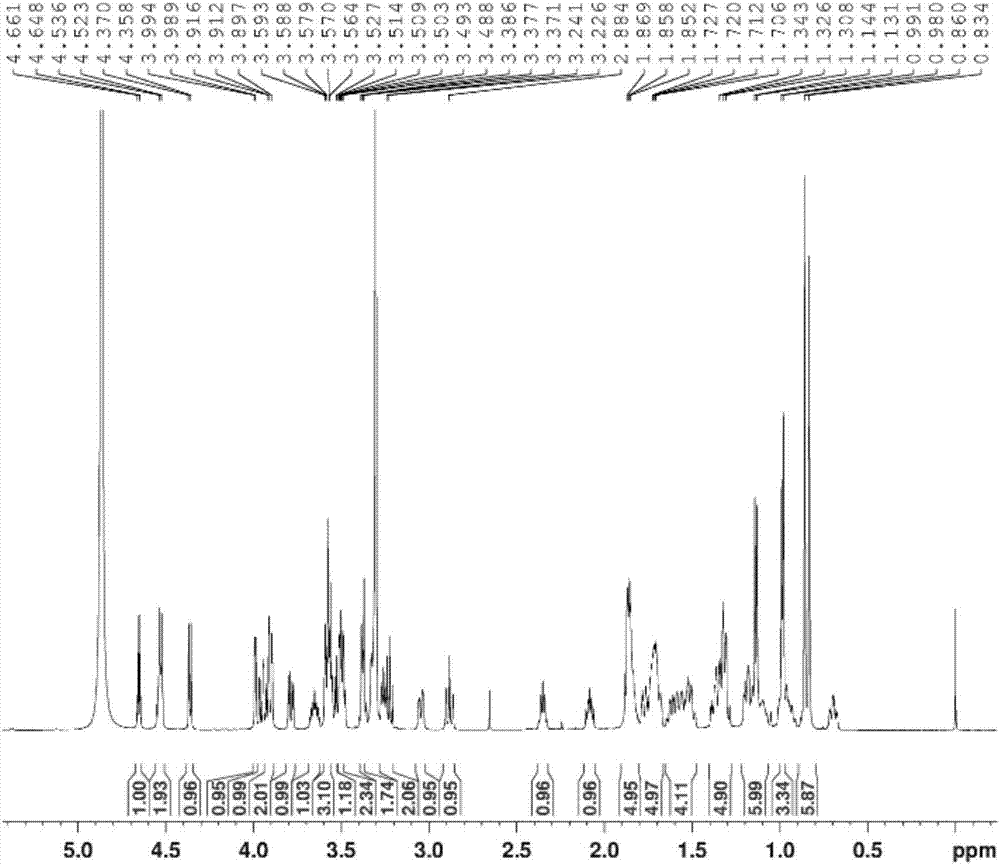
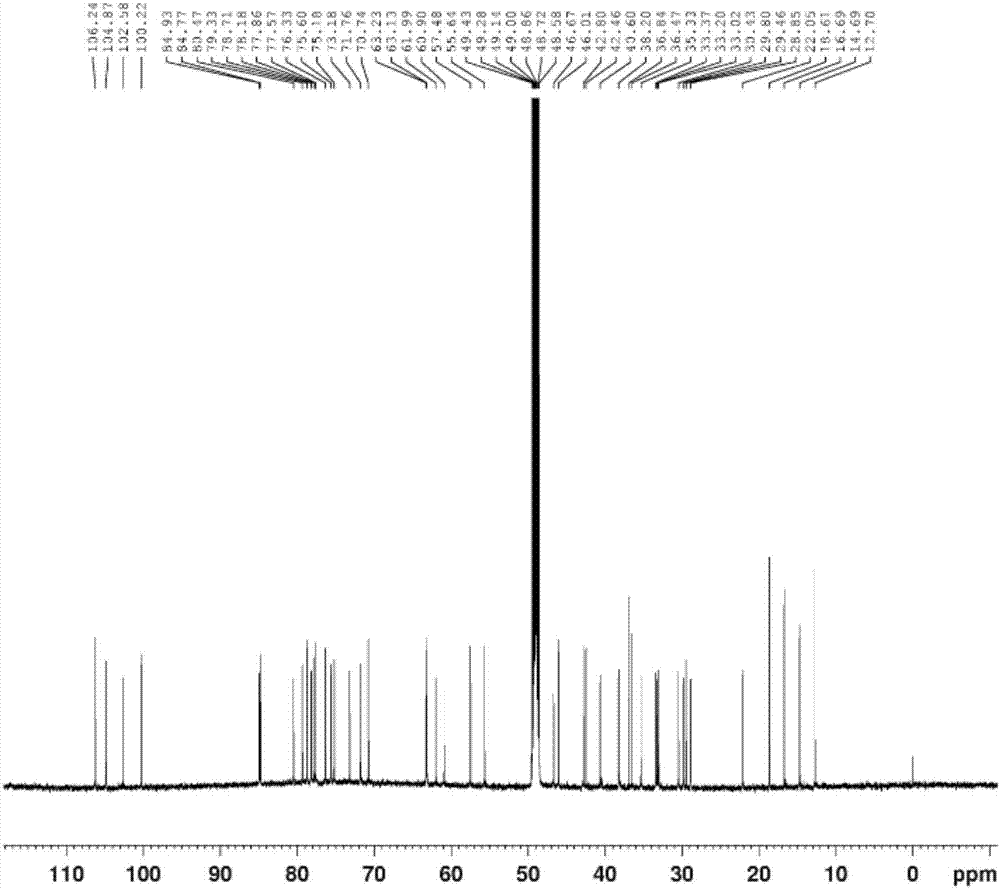
[0064] The (3β, 5α, 22α, 25R)-spirosterane-3-O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranosyl- The preparation method of (1→4)-β-D-galactopyranoside comprises the following steps:
[0065] (1) Take 10Kg of dried whole herb of Baiying, add 80L of ethanol aqueous solution with a volume concentration of 70%, reflux extraction 3 times, extract 2h each time, filter, and combine the filtrates; Extract, add distilled water 10 times the mass of the extract to disperse, filter, take the filtrate and add it to a D151 macroporous adsorption resin column, and then use 3 times the column volume of distilled water and 3 times the column volume of ethanol aqueous solution with a volume concentration of 95% to elute , discard the eluent, and then use 4 times of column volumes to elute with an ethanol aqueous solution that contains a volume fraction of 6‰ hydrochloric acid, and the volume concentration of ethanol in the hydrochloric acid ethanol aqueous solution is 95%. Neutralize to neutral, ...
Embodiment 2
[0076] The (3β, 5α, 22α, 25R)-spirosterane-3-O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranosyl- The preparation method of (1→4)-β-D-galactopyranoside comprises the following steps:
[0077] (1) Refined total alkaloids of Baiying: take 10Kg dried whole herb of Baiying, add 60L volume concentration of 70% ethanol aqueous solution, reflux extraction 4 times, extract 2h each time, filter, and combine the filtrates; concentrate the above filtrates to 50°C For the extract with a relative density of 1.05, add distilled water 8 times the mass of the extract to disperse, filter, take the filtrate and add it to a D151 macroporous adsorption resin column, and use 2 times the column volume of distilled water and 4 times the column volume to make the volume concentration 95%. Elute with ethanol aqueous solution, discard the eluent, and then use 3 times of the column volume to elute with ethanol aqueous solution containing hydrochloric acid with a volume concentration of 6‰, the volume concentr...
Embodiment 3
[0083] The (3β, 5α, 22α, 25R)-spirosterane-3-O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranosyl- The preparation method of (1→4)-β-D-galactopyranoside comprises the following steps:
[0084] (1) Refined total alkaloids of Baiying: take 10Kg dried whole herb of Baiying, add 100L volume concentration of 70% ethanol aqueous solution, reflux extraction 2 times, extract 2h each time, filter and combine the filtrates; concentrate the above filtrates to 50°C For the extract with a relative density of 1.05, add distilled water 12 times the mass of the extract to disperse, filter, take the filtrate and add it to a D151 macroporous adsorption resin column, and use 4 times the column volume of distilled water and 2 times the column volume to make the volume concentration 95%. Elute with ethanol aqueous solution, discard the eluent, and then use 5 times of column volumes to elute with ethanol aqueous solution that contains a volume fraction of 6‰ hydrochloric acid, the volume concentration o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
| Half inhibitory concentration | aaaaa | aaaaa |
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com