A kind of spirosterine alkaloid glycoside alkaloid and its preparation method and use
A technology for spiro steroid alkane and alkaloid, which is applied in the field of glycoside alkaloid preparation, can solve the problems of incomplete confirmation of component efficacy and mutual influence, complex components, etc., and achieves the effect of exact function and single component.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
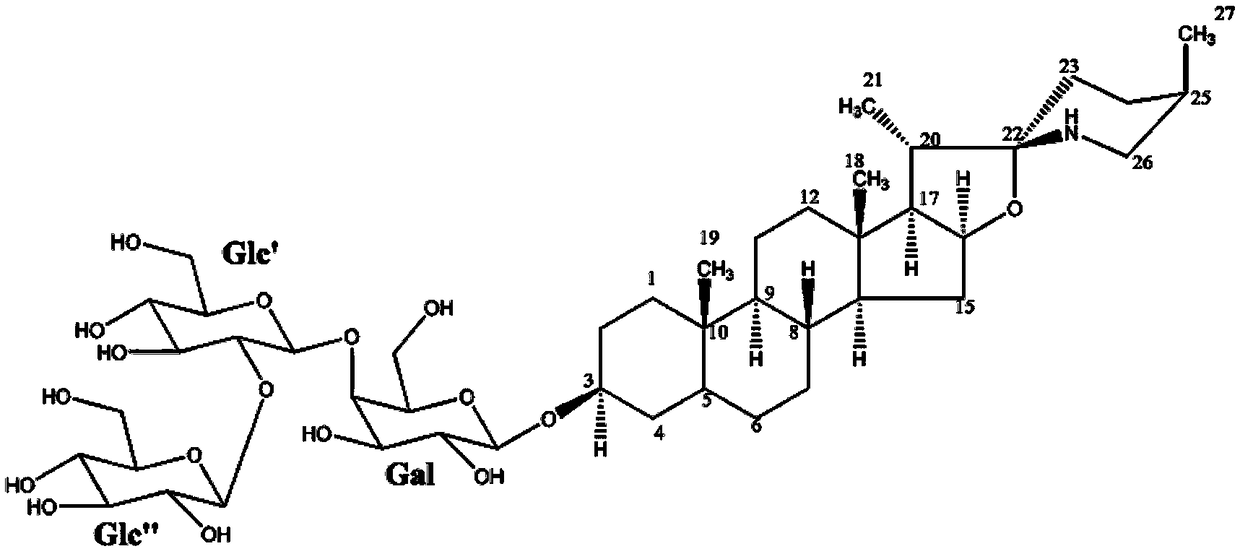
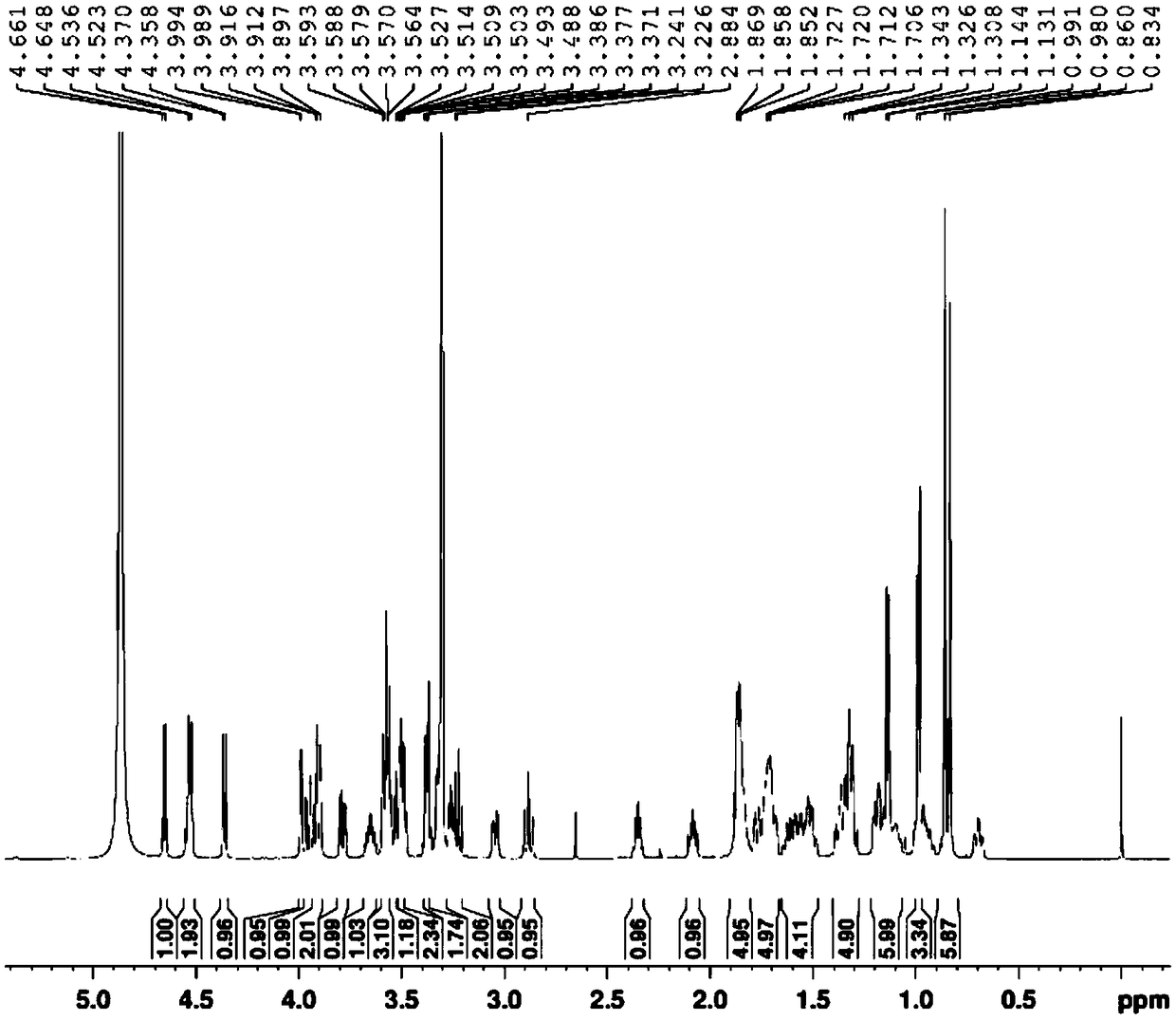
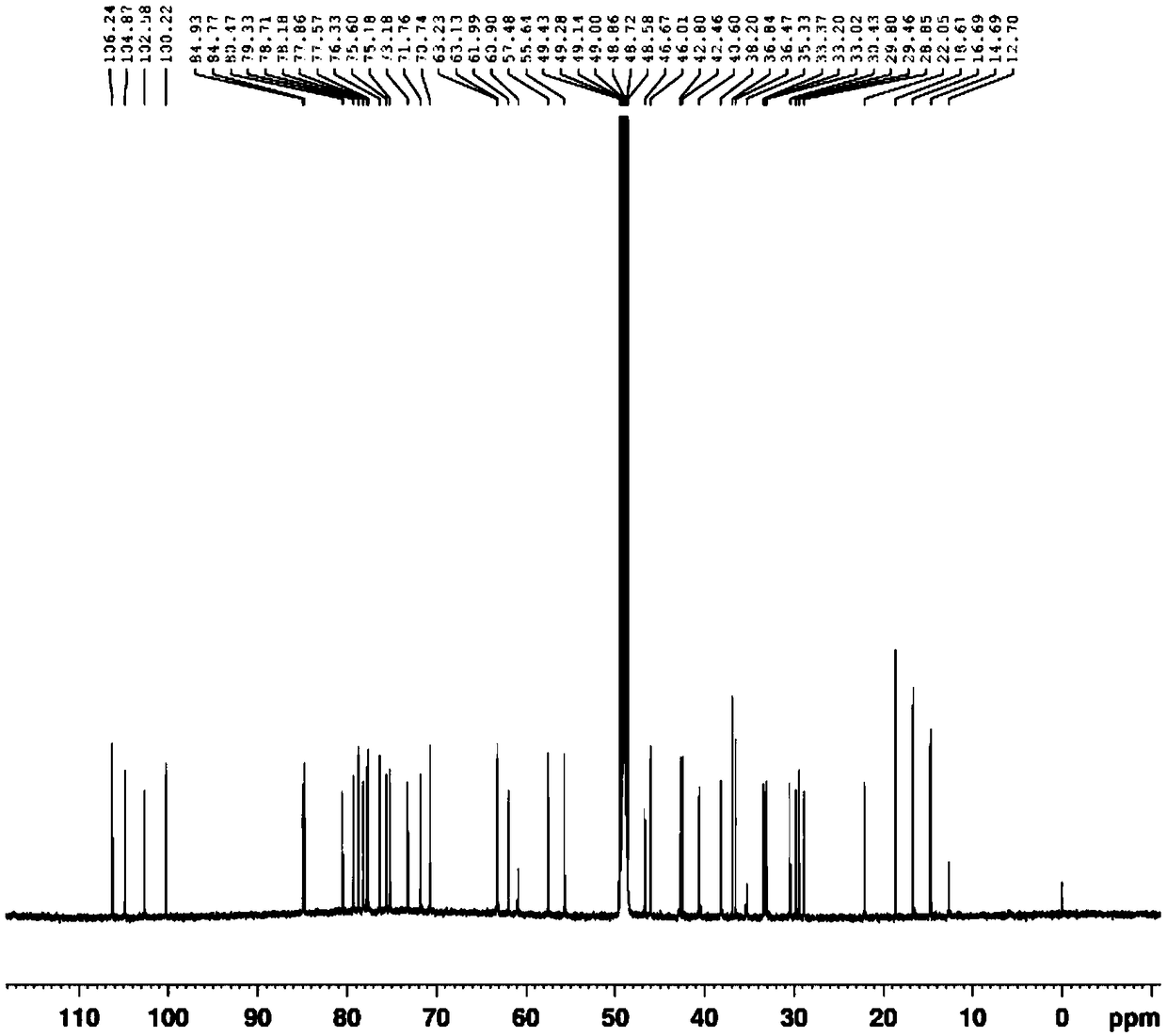
[0064] (3β,5α,22α,25R)-spirosterine alkane-3-O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranosyl- The preparation method of (1→4)-β-D-galactopyranoside comprises the following steps:
[0065] (1) Take 10Kg dried whole grass of Baiying, add 80L of 70% ethanol aqueous solution by volume, extract by reflux for 3 times, extract 2h each time, filter, and combine the filtrates; the above filtrate is concentrated to a relative density of 1.05 at 50°C. The extract, add 10 times the quality of the extract to disperse in distilled water, filter, take the filtrate and add it to a D151 macroporous adsorption resin column, and sequentially use 3 times the column volume of distilled water and 3 times the column volume of the 95% ethanol aqueous solution to elute , discard the eluent, and then use 4 times the column volume to elute with an ethanol aqueous solution containing a volume fraction of 6‰ hydrochloric acid. The volume concentration of ethanol in the hydrochloric acid ethanol aqueous so...
Embodiment 2
[0076] (3β,5α,22α,25R)-spirosterine alkane-3-O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranosyl- The preparation method of (1→4)-β-D-galactopyranoside comprises the following steps:
[0077] (1) Refined total alkaloids of Baiying: take 10Kg of dried whole plant of Baiying, add 60L of 70% ethanol aqueous solution by volume, extract by reflux for 4 times, extract for 2 hours each time, filter, and combine the filtrates; the above filtrate is concentrated to 50 ℃ The extract with a relative density of 1.05 was added with distilled water of 8 times the mass of the extract to disperse, filtered, and the filtrate was added to a D151 macroporous adsorption resin column, and the volume concentration of 2 times the column volume of distilled water and 4 times the column volume was 95%. Elution with ethanol aqueous solution, discarding the eluent, and then eluting with an ethanol aqueous solution containing 6‰ hydrochloric acid with a volume concentration of 6‰, the volume concentration of e...
Embodiment 3
[0083] (3β,5α,22α,25R)-spirosterine alkane-3-O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranosyl- The preparation method of (1→4)-β-D-galactopyranoside comprises the following steps:
[0084] (1) Refining total alkaloids of Baiying: take 10Kg dried whole plant of Baiying, add 100L of 70% ethanol aqueous solution by volume, extract twice by reflux, extract for 2 hours each time, filter, and combine the filtrates; the above filtrate is concentrated to a temperature of 50°C The extract with a relative density of 1.05 was added with distilled water of 12 times the mass of the extract to disperse it, filtered, and the filtrate was taken and added to a D151 macroporous adsorption resin column, and the volume concentration of 4 times the column volume of distilled water and 2 times the column volume was 95%. Elution with ethanol aqueous solution, discard the eluent, and then use 5 times the column volume to elute with an ethanol aqueous solution containing a volume fraction of 6‰ hydroch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com