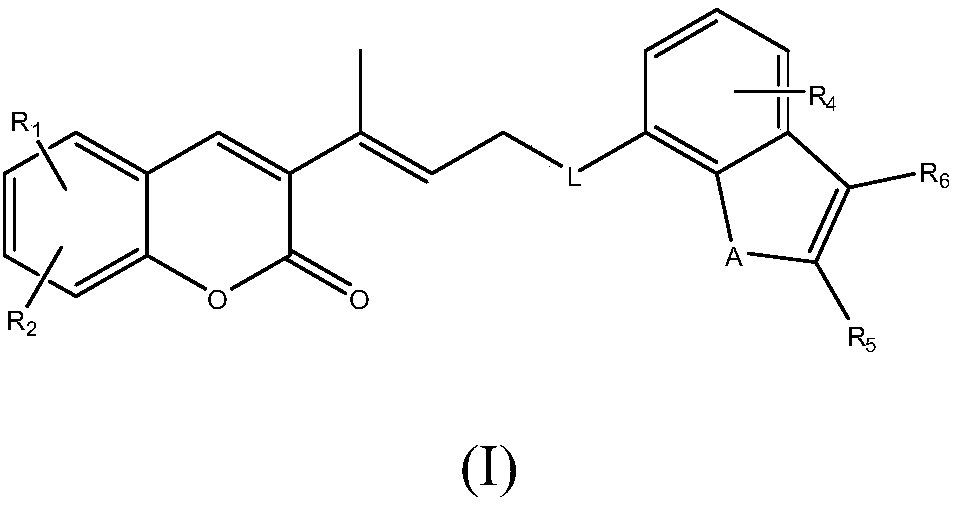
Medicament for preventing and treating stroke and preparation method thereof
A technology of stroke, medicine, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
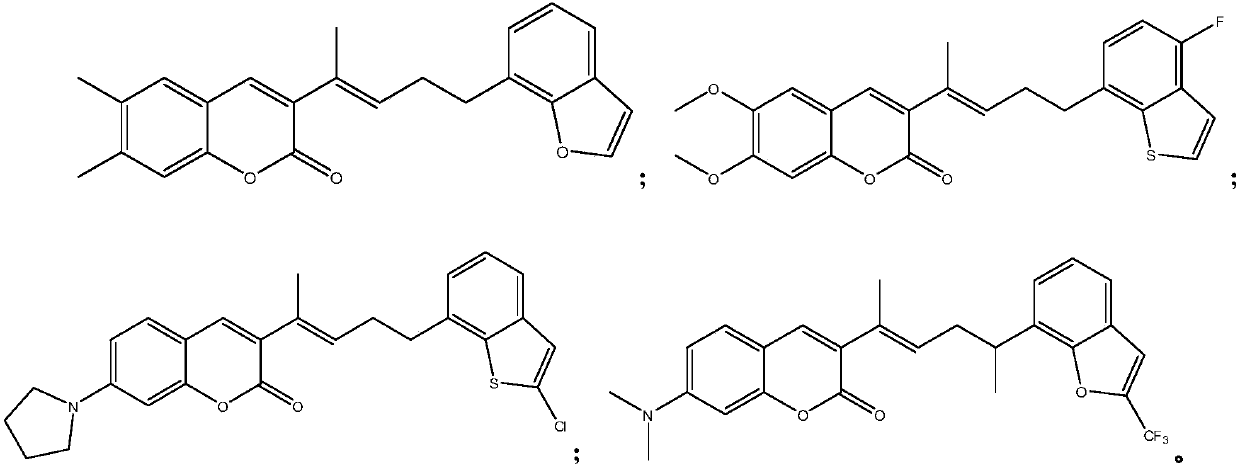
[0051] Example 1: (E)-3-(5-(benzofuran-7-yl)pent-2-en-2-yl)-6,7-dimethyl-2H-chromene-2-one ( Compound A)
[0052]
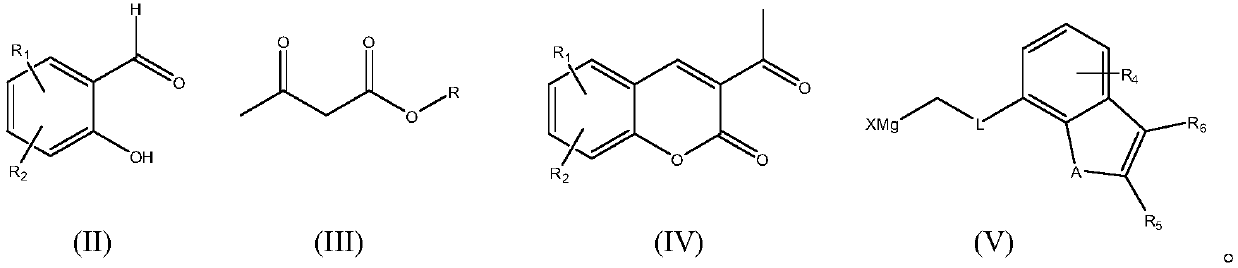
[0053] Dissolve 1.50g of 2-hydroxy-4,5-dimethylbenzaldehyde in 200ml of absolute ethanol, add 20ml of ethyl acetoacetate and 2ml of piperidine, stir and heat, a solid appears after a few minutes, reflux for 25min, and cool to room temperature After filtration, 2.01 g of the desired product 3-acetyl-6,7-dimethyl-2H-chromen-2-one was obtained, with a yield of 93.1% and a content of 96.5%. ESI-MS: 217.08[M+H] +
[0054] 1.08 g of 3-acetyl-6,7-dimethyl-2H-chromen-2-one obtained in the above steps and 0.43 g of anhydrous lithium chloride were dissolved in 40 ml of dry tetrahydrofuran, and then newly prepared ( A solution of 2-(benzofuran-7-yl)ethyl)magnesium chloride 1.53g dissolved in 30ml of tetrahydrofuran, after reflux for 2h, the reaction mixture was cooled and 20ml of saturated ammonium chloride aqueous solution was added, and the aqueous phase was washed ...
Embodiment 2
[0059] Example 2: (E)-3-(5-(4-fluoro-benzo[b]thiophen-7-yl)pent-2-en-2-yl)-6,7-dimethoxy-2H -Chromen-2-one (Compound B)
[0060]
[0061] ESI-MS: 425.11[M+H] +
[0062] Elemental analysis: theoretical value / measured value, C(67.91 / 67.78), H(4.99 / 5.07), F(4.48 / 4.57), O(15.08 / 15.01), S(7.55 / 7.57)
[0063] 1 H NMR (400MHz, CDCl 3 )δ7.78(d,1H),7.74(d,1H),7.59(s,1H),7.31(d,1H),7.13(d,1H),6.87(s,1H),6.72(s,1H ), 5.42(m,1H), 3.82(s,6H), 2.56(t,2H), 2.28(m,2H), 2.12(d,3H).
Embodiment 3
[0064] Example 3: (E)-3-(5-(2-chloro-benzo[b]thiophen-7-yl)pent-2-en-2-yl)-7-(pyrrolidin-1-yl) -2H-chromen-2-one (Compound C)
[0065]
[0066] ESI-MS: 450.12[M+H] +
[0067] Elemental analysis: theoretical value / measured value, C(69.40 / 69.52), H(5.38 / 5.47), Cl(7.88 / 7.79), N(3.11 / 3.08), O(7.11 / 7.14), S(7.13 / 7.00)
[0068] 1 H NMR (400MHz, CDCl 3 )δ7.62(d,1H),7.59(s,1H),7.47-7.32(m,3H),7.02(s,1H),6.67(d,1H),6.51(s,1H),5.43(m ,1H), 3.42(t,4H), 2.56(t,2H), 2.28(m,2H), 2.12(d,3H), 1.82(m,4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com