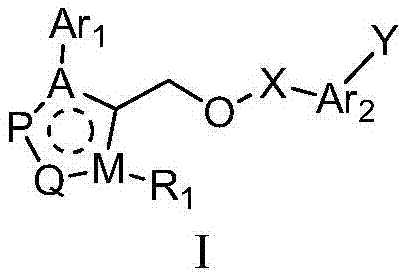
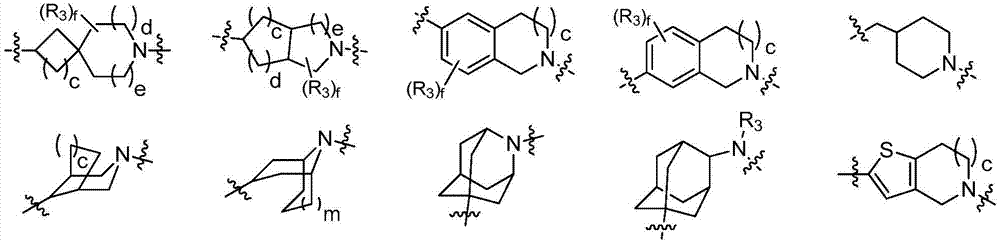
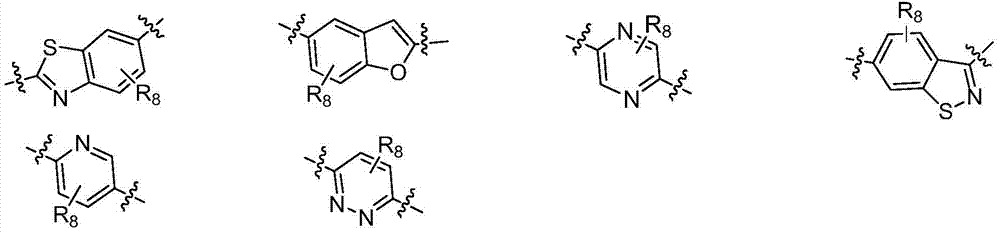
FXR receptor modulator, and preparation method and application thereof
A receptor modulator and solvate technology, applied in the field of FXR receptor modulator and its preparation, can solve the problem of not activating human or mouse receptors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] 3-{2-{[5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-yl]methoxy}-7-azaspiro[3.5]nonane-7 - Preparation of carbonyl}benzoic acid.
[0138]
[0139] Prepare according to the following lines:
[0140]
[0141] (1) Preparation of 2,6-dichlorobenzaldehyde oxime.
[0142] Dissolve 2,6-dichlorobenzaldehyde (5.0g, 28.6mmol) in absolute ethanol (45mL), stir at room temperature, and add NH 2 OH·HCl (2.3 g, 33.1 mmol), NaOH (1.3 g, 32.5 mmol) and water (20 mL), refluxed overnight in an oil bath at 90°C. After the reaction solution was cooled to room temperature, the solvent was spin-dried, water (100 mL) was added, extracted with ethyl acetate (2×100 mL), the combined organic layers were washed with saturated brine (2×100 mL), anhydrous Na 2 SO 4 Dry, filter, spin dry, add petroleum ether (100 mL), stir well, filter, and dry to obtain 3.5 g of white solid 2,6-dichlorobenzaldehyde oxime, yield: 64%.
[0143] (2) Preparation of chlorinated 2,6-dichlorobenzaldehyde oxime....
Embodiment 2
[0174] 2-{2-{[5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-yl]methoxy}-7-azaspiro[3.5]nonane-7 Preparation of -yl}benzo[d]thiazole-6-carboxylic acid.
[0175]
[0176] (1) Preparation of ethyl 2-aminobenzo[d]thiazole-6-carboxylate.
[0177] Dissolve ethyl 4-aminobenzoate (4.9g, 30.0mmol), potassium thiocyanate (11.6g, 120.0mmol) in acetic acid (180mL), add bromine (1.5mL, 30.0mmol) dropwise at 19°C Acetic acid (20 mL) solution was added over 20 minutes, and then raised to room temperature to react overnight. Pour the reaction solution into water (100mL), stir for 10 minutes, add ammonia water dropwise to adjust the pH to about 8, filter, wash the filter cake with water and petroleum ether successively, and dry in vacuo to obtain 6.7g of a yellow solid, which is 2- Ethyl aminobenzo[d]thiazole-6-carboxylate, yield: 100%.
[0178] The characterization data is: 1 H NMR (400MHz, DMSO) δ: 8.28(d, J=1.6Hz, 1H), 7.88(s, 2H), 7.82(dd, J=1.6Hz, 8.4Hz, 1H), 7.37(d, J=8.4 Hz, 1H...
Embodiment 3
[0189] 3-{6-{[5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol4-yl]methoxy}2-azaspiro[3.3]heptane-2-carbonyl } Preparation of benzoic acid.
[0190]
[0191] (1) Preparation of tert-butyl 6-hydroxy-2-azaspiro[3.3]heptane-2-carboxylate.
[0192] The method for preparing 2-hydroxyl-7-aza[3.5]nonane-7-carboxylate tert-butyl with reference to Example 1 prepares 6-hydroxyl-2-azaspiro[3.3]heptane-2-carboxylate tert-butyl, Only tert-butyl 4-oxopiperidine-1-carboxylate was replaced by tert-butyl 3-oxazetidine-1-carboxylate.
[0193] Product characterization data are: 1 H NMR (400MHz, CDCl 3 )δ:4.20(m,1H),3.90(s,2H),3.89(s,2H),2.55(m,2H),2.09(m,2H),1.95(m,1H),1.44(s,9H ).
[0194] (2) 3-{6-{[5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol4-yl]methoxy}2-azaspiro[3.3]heptane- Preparation of 2-carbonyl}benzoic acid.
[0195] Preparation of 3-{2-{[5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol4-yl]methoxy}-7-azaspiro[3.5] with reference to Example 1 Preparation of 3-{6-{[5-cyclopropyl-3-(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com