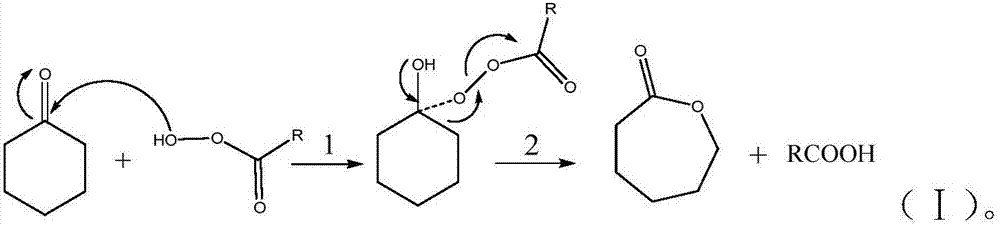
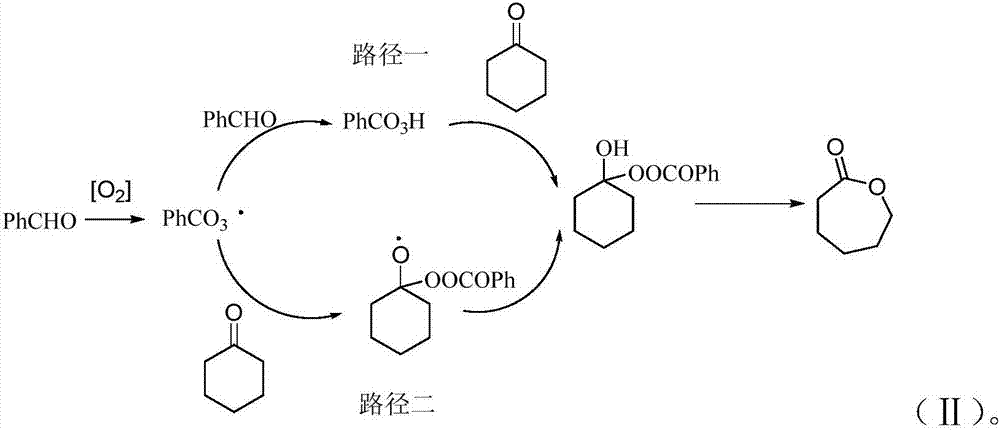
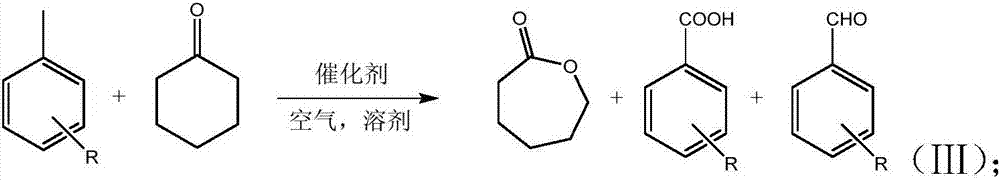
Novel method for preparing epsilon-caprolactone
A technology of caprolactone and cyclohexanone, which is applied in the field of preparing ε-caprolactone, can solve the problems of lack of industrialization value, high process cost and high price of benzaldehyde, and achieves the advantages of low cost, easy availability and reduced production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add Fe(NO 3 ) 3 ·9H 2 O 0.323kg, NHPI 0.326kg, toluene 1.840kg, cyclohexanone 0.980kg, acetonitrile 20L. Pour in air, adjust the pressure to 0.2MPa, set the temperature to 40°C, and stir. Start the timing reaction, continue the reaction for 10h, stop the reaction, wait for the reaction solution to cool to room temperature, distill out the acetonitrile solvent under reduced pressure, filter NHPI, Fe(NO) 3 ) 3, washed three times with acetonitrile, and dried to recover the catalyst. Benzaldehyde and benzoic acid were separated by vacuum distillation to obtain 0.602 kg of cyclohexanone, 0.403 kg of ε-caprolactone, 0.090 kg of toluene, 0.464 kg of benzaldehyde and 1.76 kg of benzoic acid. The conversion of cyclohexanone was 38.6%, and the selectivity of ε-caprolactone was 91.5%. The conversion of toluene was 95.1%, the selectivity of benzaldehyde was 23%, and the selectivity of benzoic acid was 75.9%.
Embodiment 2
[0051] Add Fe(NO 3 ) 3 ·9H 2 O 0.323kg, NHPI 0.326kg, toluene 1.840kg, cyclohexanone 0.980kg, ethyl acetate 20L. Pour in air, adjust the pressure to 0.2MPa, set the temperature to 60°C, and stir. Start the timing reaction, continue the reaction for 10h, stop the reaction, wait for the reaction solution to cool to room temperature, distill out the ethyl acetate solvent under reduced pressure, filter NHPI, Fe(NO) 3 ) 3 , washed three times with acetonitrile, and dried to recover the catalyst. Benzaldehyde and benzoic acid were separated by vacuum distillation to obtain 0.546kg of cyclohexanone, 0.465kg of ε-caprolactone, 0.160kg of toluene, 0.472kg of benzaldehyde and 1.357kg of benzoic acid. The conversion of cyclohexanone was 44.3%, and the selectivity of ε-caprolactone was 92.1%. Toluene conversion was 91.3%, benzaldehyde selectivity was 24.4%, and benzoic acid selectivity was 61.0%
Embodiment 3
[0053] Add Fe(NO 3 ) 3 ·9H 2 O 0.323kg, NHPI 0.326kg, toluene 1.840kg, cyclohexanone 0.980kg, dichloroethane 20L. Pour in air, adjust the pressure to 0.2MPa, set the temperature to 60°C, and stir. Start the timing reaction, continue the reaction for 10h, stop the reaction, wait for the reaction solution to cool to room temperature, distill out the dichloroethane solvent under reduced pressure, filter NHPI, Fe(NO) 3 ) 3 , washed three times with acetonitrile, and dried to recover the catalyst. Benzaldehyde and benzoic acid were separated by vacuum distillation to obtain 0.496kg of cyclohexanone, 0.541kg of ε-caprolactone, 0.168kg of toluene, 0.406kg of benzaldehyde and 1.63kg of benzoic acid. The conversion of cyclohexanone was 49.4%, and the selectivity of ε-caprolactone was 96.1%. Toluene conversion was 90.8%, benzaldehyde selectivity was 21.1%, and benzoic acid selectivity was 73.6%
[0054] From Examples 1-3, it can be seen that, compared with acetonitrile and ethyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com