Fluorine ion adsorbent with high adsorbing capacity and preparation method thereof
A fluoride ion and adsorbent technology, which is applied in the field of high adsorption capacity fluoride ion adsorbent and its preparation, can solve the problems of difficult control, complicated operation, long process, etc., and achieves a short production cycle, simple operation and high adsorption capacity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
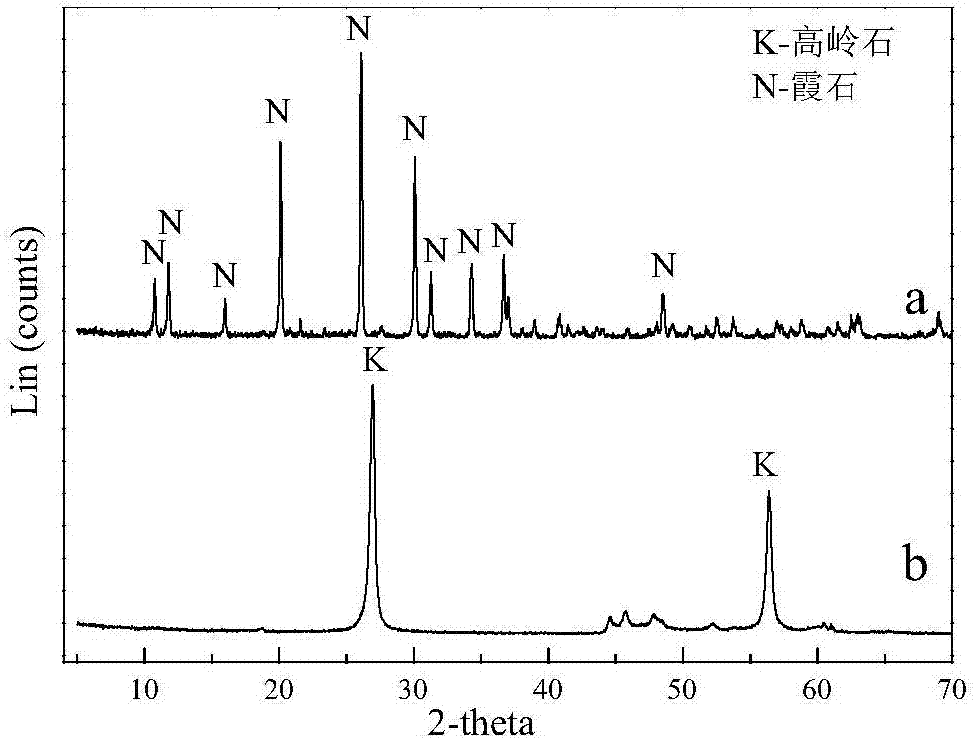
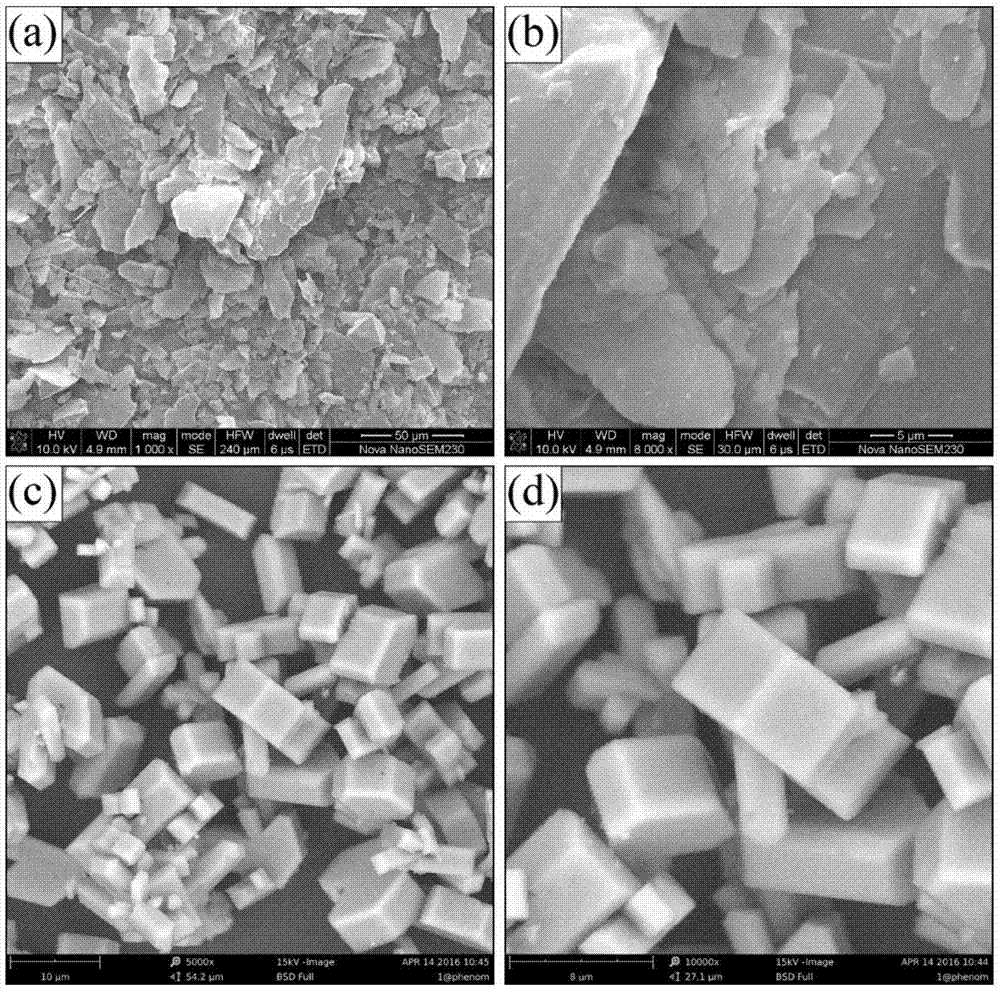
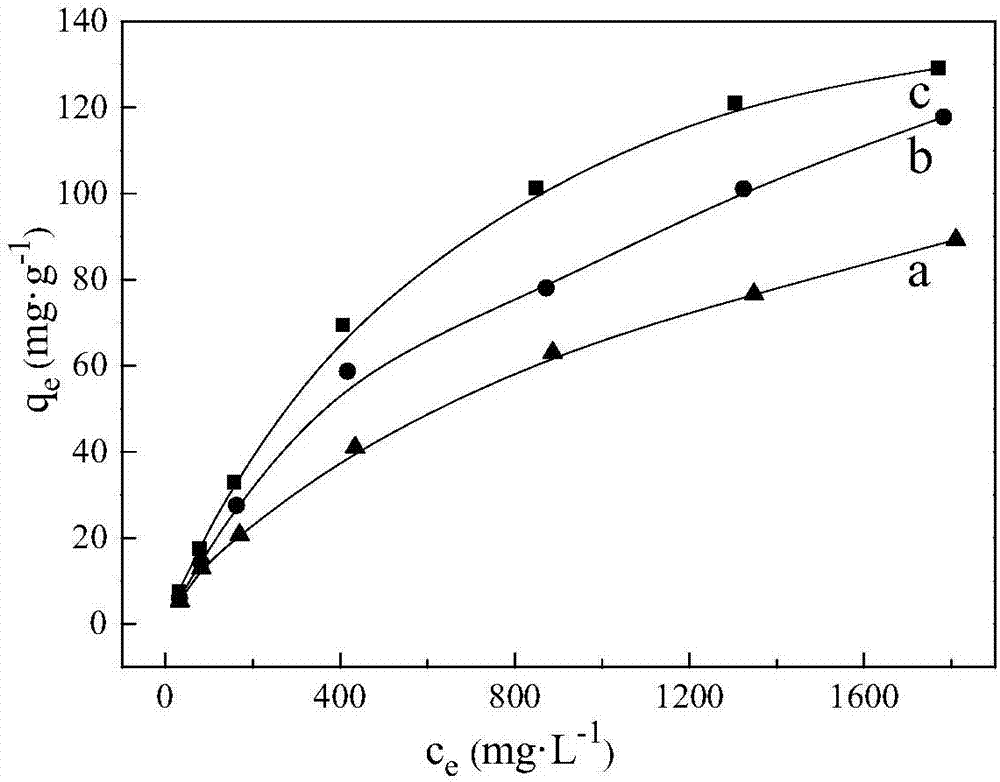
[0028] Kaolin is purified by physical methods (washing method or re-election method or flotation method), and 4g of purified kaolin minerals are weighed (see XRD analysis and scanning electron microscope photos for figure 1 and figure 2 ), the content of kaolinite in this kaolin mineral is greater than 95%, and the particle size distribution is 15-20 μm. It was placed in a high-pressure reactor, and then 100 mL of deionized water and 4 g of sodium hydroxide powder were added to react for 2 hours at 240 ° C. During the reaction, the stirring speed was 1000 rpm until the hydrothermal reaction was completed. Subsequently, the solid-liquid mixture after the reaction is subjected to solid-liquid separation by means of suction filtration. The separated solid was added to deionized water to wash immediately, and the washing temperature was 90° C., and the washing was repeated three times. The washed product was placed in a 60°C environment until it was completely dry, and the XRD ...
Embodiment 2
[0030] Kaolin was purified by physical method (washing method or re-election method or flotation method), and 4g of kaolin mineral (same as that used in Example 1) was weighed. It was placed in a high-pressure reactor, and then 100 mL of deionized water and 4 g of sodium hydroxide powder were added to react for 1 h at 260 ° C. During the reaction, the stirring speed was 900 rpm until the hydrothermal reaction was completed. Subsequently, the reacted solid-liquid mixture is subjected to solid-liquid separation by centrifugation. The separated solid was then washed with deionized water at a temperature of 95° C., and the washing was repeated three times. The washed product was placed in an environment of 70 °C until it was completely dry, and finally a nepheline-type adsorbent sample was obtained. The specific surface area of the nepheline-type adsorbent material was 18.5m 2 / g. The main chemical composition of the fluoride ion adsorbent is Al 2 o 3 , SiO 2 , Na 2 O and ...
Embodiment 3
[0032] Kaolin was purified by physical method (washing method or re-election method or flotation method), and 4g of kaolin mineral (same as that used in Example 1) was weighed. It was placed in a high-pressure reactor, and then 100 mL of deionized water and 4 g of sodium hydroxide powder were added, and reacted at 220 ° C for 3 h. During the reaction, the stirring speed was 500 rpm until the hydrothermal reaction was completed. Subsequently, the solid-liquid mixture after the reaction is subjected to solid-liquid separation by means of pressure filtration. The separated solid was then washed with deionized water at a temperature of 90° C., and the washing was repeated four times. The washed product is placed in an environment of 80°C until it is completely dry, and finally a nepheline-type adsorbent sample is obtained. The specific surface area of the nepheline-type adsorbent material is 15.2m 2 / g. The main chemical composition of the fluoride ion adsorbent is Al 2 o 3 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com