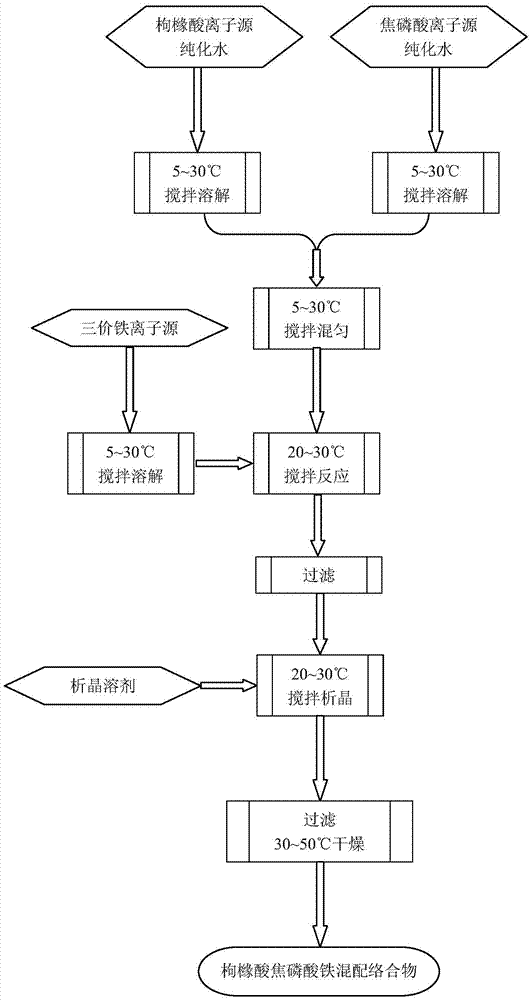
Ferric pyrophosphate citrate mixed complex preparation method
A technology of ferric citrate pyrophosphate and complexes, which is applied in the field of medicine, can solve problems such as difficulty in guaranteeing product quality and poor stability, and achieve the effects of low content of organic impurities, reduction of content, and avoidance of damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
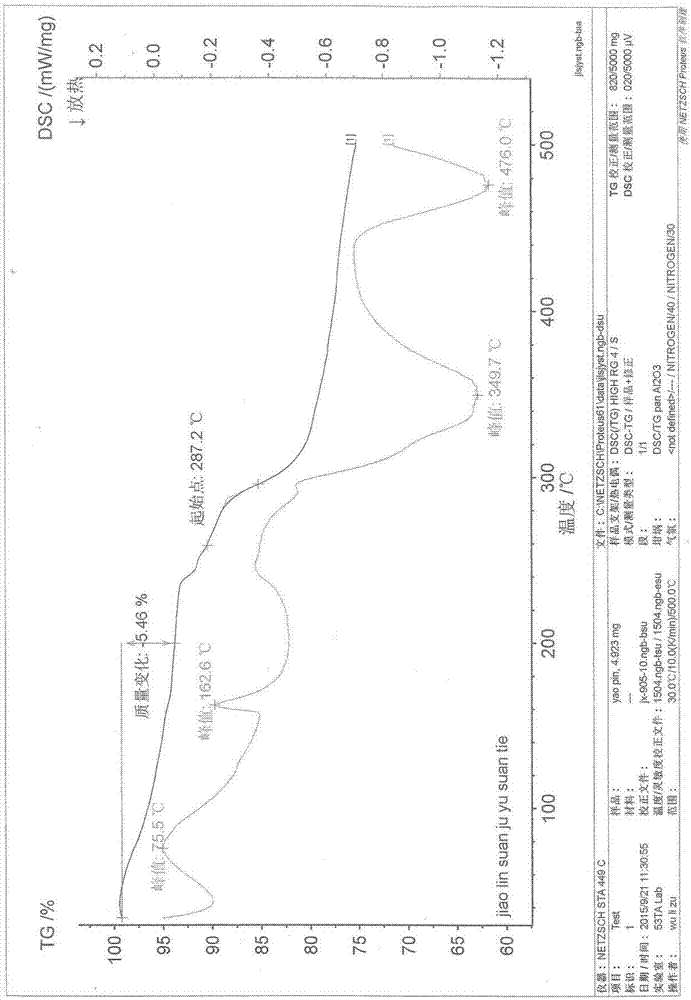
Image
Examples
Embodiment 1
[0052] Ferric cation content is 25.23g (Fe 2 (SO 4 ) 3 ·xH 2 O, 0.05mol) was added to the reaction flask I, 126.2g of purified water was added, and stirred at 25°C for 12 hours, the solid was completely dissolved, and it was a light yellow clear solution.
[0053] Sodium citrate 29.41g (Na 3 C 6 h 6 o 7 2H 2 (2, MW294.1, 0.1mol) was added to reaction flask II, 88.3g of purified water was added, stirred for 1 hour at 25°C, and completely dissolved to obtain a colorless and clear solution; 44.61g of sodium pyrophosphate (Na 4 P 2 o 7 10H 2 O, MW446.06, 0.1 mol) was added to reaction flask III, then 669.2 g of purified water was added, stirred at 25° C. for 1 hour, and completely dissolved to obtain a colorless and clear solution. Slowly add the completely dissolved sodium pyrophosphate aqueous solution into the sodium citrate aqueous solution, and stir at 25°C for 2 hours.
[0054] The ferric sulfate solution was added dropwise to the mixed solution under stirring, a...
Embodiment 2
[0068] Ferric iron content is 22.2% ferric sulfate hydrate 25.23g (Fe 2 (SO 4 ) 3 ·xH 2 O, 0.05mol) was added to the reaction flask I, 176.6g of purified water was added, and stirred at 5°C for 16 hours, the solid was completely dissolved, and a light yellow clear solution was obtained.
[0069] Sodium citrate 29.41g (Na 3 C 6 h 6 o 7 2H 2 (2, MW294.1, 0.1mol) into the reaction flask II, add 58.8g of purified water, stir and dissolve at 5°C to obtain a colorless and clear solution; add 44.61g of sodium pyrophosphate (Na 4 P 2 o 7 10H 2 O, MW446.06, 0.1 mol) was added to reaction flask III, then 535.3 g of purified water was added, stirred at 5°C for 1 hour, and completely dissolved to obtain a colorless and clear solution. Slowly add the completely dissolved sodium pyrophosphate aqueous solution into the sodium citrate aqueous solution, and stir at 5°C for 2 hours.
[0070] The ferric sulfate solution prepared was added dropwise to the mixed solution under stirring...
Embodiment 3
[0074] Ferric iron content is 22.2% ferric sulfate hydrate 25.23g (Fe 2 (SO 4 ) 3 ·xH 2 O, 0.05mol) was added to the reaction flask I, 126.2g of water was added, and stirred at 20°C for 15 hours, the solid was completely dissolved, and it was a light yellow clear solution.
[0075] Sodium citrate 29.41g (Na 3 C 6 h 6 o 7 2H 2 (2, MW294.1, 0.1mol) into the reaction bottle II, add 88.3g of purified water, stir at 20°C for 1 hour, dissolve completely, and obtain a colorless and clear solution; add 35.69g of sodium pyrophosphate (Na 4 P 2 o 7 10H 2 O, MW446.06, 0.08mol) was added to reaction flask III, then 535g of purified water was added, stirred at 20°C for 1 hour, and completely dissolved to obtain a colorless and clear solution. Slowly add the completely dissolved sodium pyrophosphate aqueous solution into the sodium citrate aqueous solution, and stir at 20°C for 2 hours.
[0076] The ferric sulfate solution prepared was added dropwise to the mixed solution under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com