Oxyntomodulin (OXM) analogue and application thereof
A technology of oxyntomodulin and analogs, applied in hormone peptides, drug combinations, peptide-nucleic acid and other directions, can solve the problem of weak hypoglycemic activity, achieve balanced hypoglycemic and weight loss activities, short synthesis cycle, and save costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
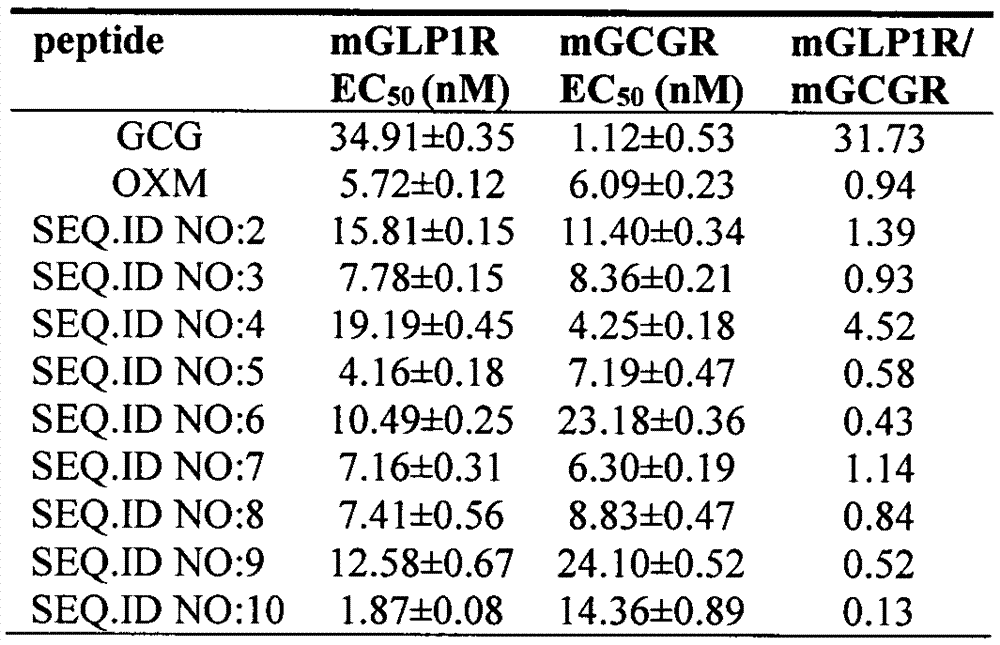
[0045] His-Ser-Gln-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Ser-Arg-Arg-Ala-Gln-Asp-Phe-Val-Gln-Trp- Leu-Met-Asn-Thr-Lys-Arg-Asn-Arg-NH 2 (SEQ. ID NO: 2);
[0046] solid phase synthesis
[0047] (1) Swelling of the resin
[0048] Weigh 50 mg of Fmoc-Rink amide-MBHA Resin (degree of substitution 0.4 mmol / g), swell with 7 mL of DCM for 30 min, filter to remove DCM, then swell with 10 mL of NMP for 30 min, and finally rinse with NMP, DCM, and 7 mL of NMP respectively.
[0049] (2) Removal of Fmoc protecting group
[0050] Put the swollen resin into the reactor, add 7mL of 25% piperidine / NMP (V / V) solution containing 0.1M HOBt, and react in the solid-state reactor for 1min, the reaction temperature is controlled within 50°C, and the Compress the air with the compressor to cool, and filter the solution after the reaction; then add 7 mL of 25% piperidine / NMP (V / V) solution containing 0.1M HOBt, and react in a solid-phase reactor for another 4 min, and the reaction tempera...
Embodiment 2~9
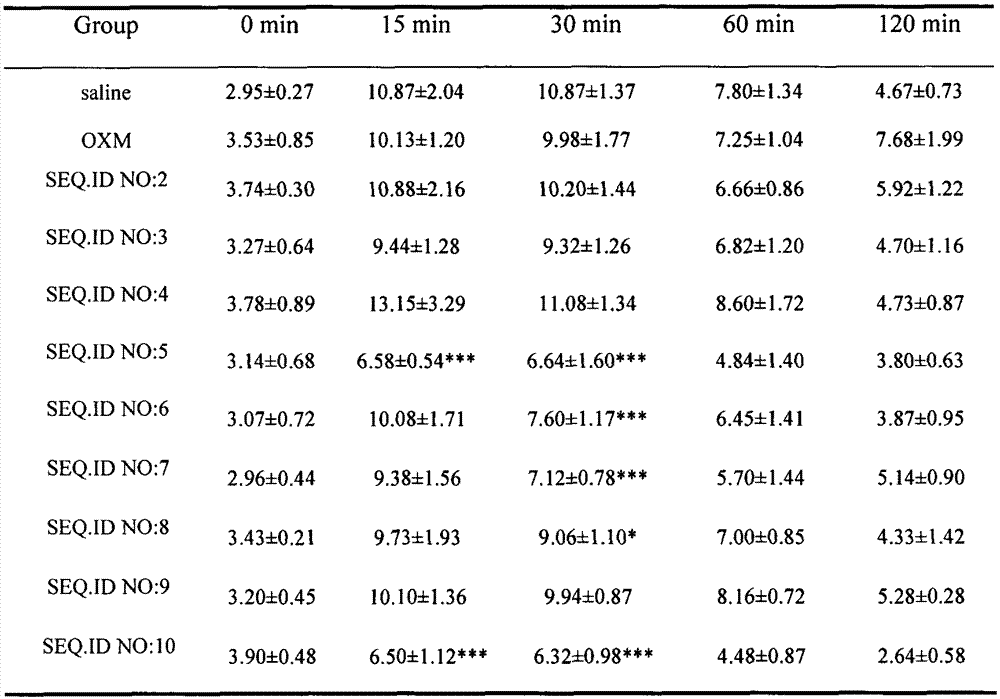
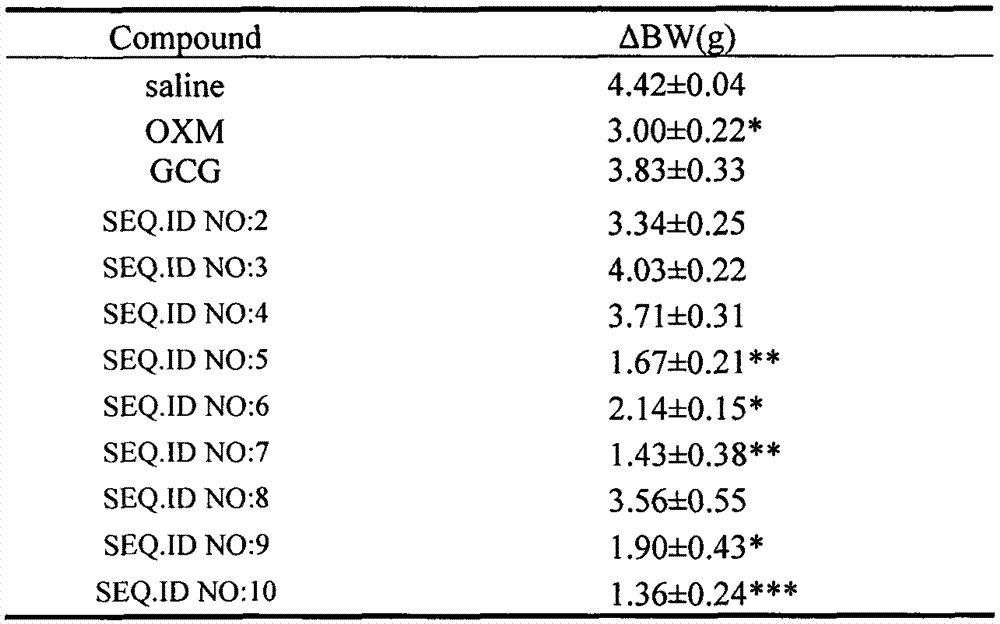
[0060] According to the method described in Example 1, the oxyntomodulin (OXM) analogs of Examples 2-9 were synthesized according to the corresponding sequences, and their respective molecular weights were confirmed by electrospray mass spectrometry (ESI-MS).
Embodiment 2
[0062] His-Ser-Gln-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Ser-Arg-Arg-Ala-Gln-Asp-Phe-Val-Gln-Trp- Leu-Met-Asn-Thr-Val-Lys-Gly-Arg-NH 2 (SEQ. ID NO: 3);
[0063] The theoretical relative molecular mass is 3922.4. ESI-MS m / z: found[M+3H] 3+ 1308.5, [M+4H] 4+ 981.5; calc.
[0064] [M+3H] 3+ 1308.4, [M+4H] 4+ 771.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com