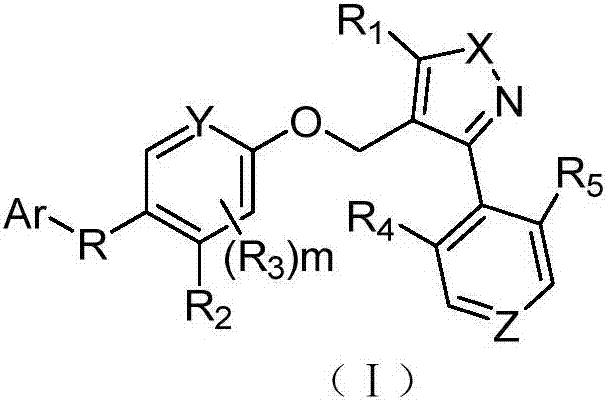
FXR agonist and preparation method and application thereof
A NR10R11, selected technology, applied in antiviral agents, pharmaceutical formulations, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0247] Example 1 3-(3-(2-chloro-4-((5-cyclopropyl-3-(2,6-dichlorophenyl)isothiazol-4-yl)methoxy)phenyl)- 3-Hydroxycyclobutyl)benzoic acid (1)
[0248]
[0249] Step 1: Dissolve compound 1-a (468mg, 1.45mmol) in dry THF (6mL), stir under nitrogen atmosphere, add n-butyl lithium (1.6M, 1.0mL) dropwise at -70~-78°C ). Stir at this temperature for 45 min after the addition. Then a solution of compound I-4 (270 mg, 1.32 mmol) in dry THF (0.5 mL) was added dropwise. Stir at this temperature for 1 h after addition. Warm to room temperature slowly, and wash with saturated NH 4 Quenched with Cl (5 mL) and extracted with ethyl acetate. The organic phase was dried, concentrated, and separated by column chromatography (PE / EA, EA: 0-15%) to obtain compound 1-b (246 mg, yield: 42%).
[0250] LC-MS:t R =3.825min,[M-OH] + = 429.1;
[0251] 1 HNMR (400MHz, CDCl 3 )δ7.97(s,1H),7.88(d,J=7.7Hz,1H),7.48(dd,J=16.4,8.1Hz,2H),7.39(t,J=7.7Hz,1H),6.96( d,J=2.5Hz,1H),6.78(dd,J=8.5,2.5Hz,1...
Embodiment 2
[0260] Example 2 3-(3-(4-((5-cyclopropyl-3-(2,6-dichlorophenyl)isothiazol-4-yl)methoxy)-2-methoxyphenyl) -3-Hydroxycyclobutyl)benzoic acid (2)
[0261]
[0262] The first step: the synthesis of compound 2-a refers to the preparation method of the first step to the third step of Example 1.
[0263] LC-MS:t R =3.465min,[M-OH] + = 592.0;
[0264] 1 HNMR (400MHz, CDCl 3 )δ7.98-7.93(m,1H),7.89-7.84(m,1H),7.53-7.44(m,1H),7.41-7.35(m,3H),7.33-7.27(m,2H),6.48- 6.35(m,2H),4.92(s,2H),3.91(s,3H),3.85(s,3H),3.05-2.89(m,3H),2.54-2.47(m,2H),2.34-2.25( m,1H), 1.33-1.26(m,2H), 0.98-0.92(m,2H).
[0265] Second step: Compound 2-a (30 mg, 0.05 mmol) was dissolved in THF / MeOH (1 mL, 1:1). Aqueous NaOH (1.0M, 1 mL) was added. The reaction solution was stirred overnight at 60 °C. After cooling, it was acidified to pH=5 with 3N HCl, then extracted twice with ethyl acetate. The organic phase was dried, concentrated, and purified by PTLC to obtain compound 2 (3.0 mg);
[0266] LC-MS:t ...
Embodiment 3
[0268] Example 3 3-(3-(4-((5-cyclopropyl-3-(2,6-dichlorophenyl)isothiazol-4-yl)methoxy)-2-methylphenyl) -3-Hydroxycyclobutyl)benzoic acid (3)
[0269]
[0270] The first step: the synthesis of compound 3-a refers to the preparation method of the first step to the third step of Example 1.
[0271] LC-MS:t R =3.514min,[M+H] + = 594.1.
[0272] Step 2: Dissolve compound 3-a (8mg, 0.013mmol) in tetrahydrofuran (1mL), add aqueous sodium hydroxide solution (1mL, 1N), and react at 60°C for 4h. After the reaction is complete, add dilute hydrochloric acid to acidify to weak acidity, and extract with ethyl acetate. The organic phase was dried, concentrated, and separated by PTLC to obtain compound 3 (4 mg, 53%).
[0273] LC-MS:t R =3.2min,[M-OH] + =580.0,582.1;
[0274] 1 HNMR (400MHz, CDCl 3 )δ7.95(s,1H),7.87(d,J=7.8Hz,1H),7.48-7.44(m,1H),7.35(d,J=7.7Hz,1H),7.33-7.28(m,3H ),7.25-7.21(m,1H),6.65(d,J=2.5Hz,1H),6.58(dd,J=8.5,2.7Hz,1H),4.85(s,2H),3.16-3.05(m, 2H),3.01-2.89(m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com