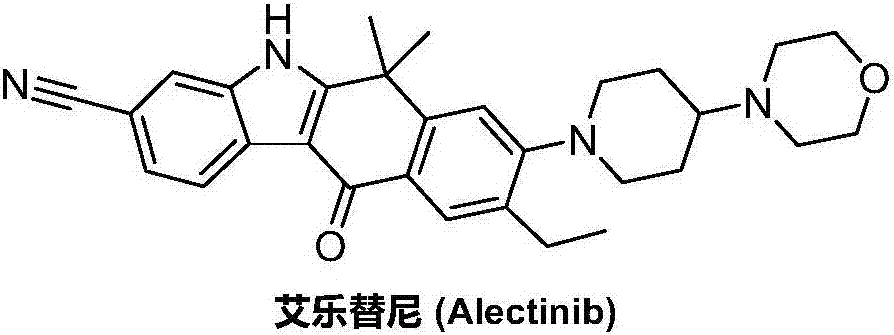
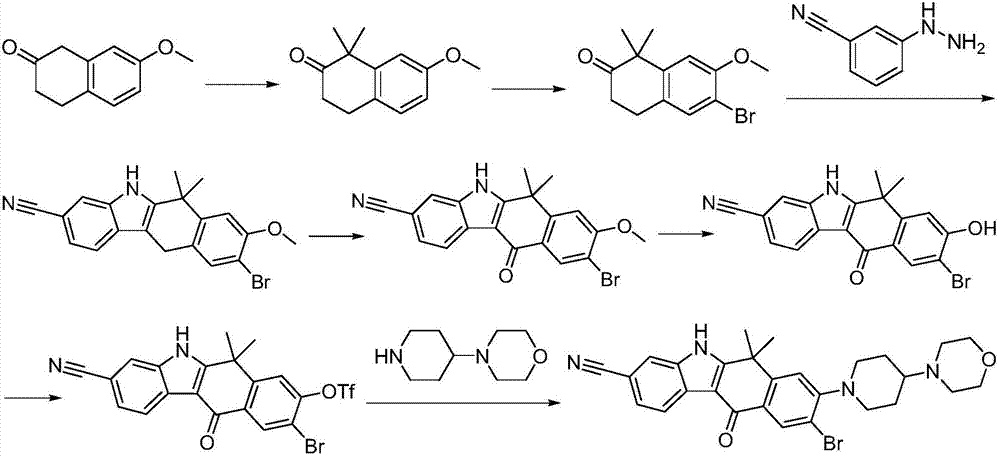
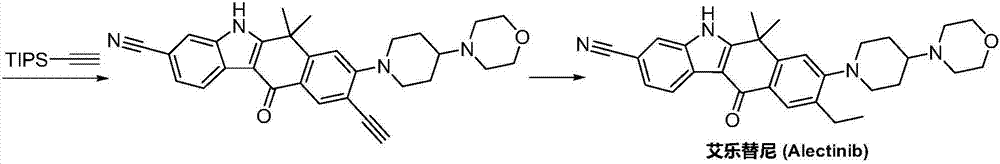
Preparation method of alectinib
A technology of alectinib and morpholine, applied in the field of pharmaceutical chemical synthesis, can solve the problems of short process flow, cumbersome operation, unfavorable industrial production and promotion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A) Preparation of 5-[(ethoxycarbonyl)methyl]-2-bromophenyl triflate:
[0048] 2-(4-Bromo-3-hydroxyphenyl)ethyl acetate (13.0g, 47.6mmol) was dissolved in triethylamine (10.2g, 100.8mmol), slowly added dropwise trifluoromethanesulfonic anhydride (19.1g, 67.7 mmol), stirred at 20°C for 2 hours, after post-treatment and purification, 5-[(ethoxycarbonyl)methyl]-2-bromophenyl trifluoromethanesulfonate was obtained as a pale yellow solid (16.6g), Yield 89%.
[0049] B) Preparation of ethyl 2-{4-bromo-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}acetate:
[0050] 5-[(ethoxycarbonyl)methyl]-2-bromophenyl trifluoromethanesulfonate (16.5g, 42.2mmol) was dissolved in N,N-dimethylformamide (250mL), and 4-( 4-piperidinyl)morpholine (16.2g, 95.2mmol), sodium methoxide (5.7g, 105.5mmol), the reaction mixture was stirred at 100°C for 12 hours, the reaction solution was cooled to room temperature, water (150mL) was added, and cooled to 0 ℃ for 4 hours, and filtered to obtain ethyl 2-{4...
Embodiment 2
[0064] A) Preparation of 5-[(ethoxycarbonyl)methyl]-2-bromophenyl triflate:
[0065] 2-(4-Bromo-3-hydroxyphenyl)ethyl acetate (3.0g, 11.0mmol) was dissolved in N,N-diisopropylethylamine (2.4g, 18.6mmol), slowly added dropwise trifluoromethyl Sulfonic anhydride (4.1g, 14.5mmol), reacted with stirring at 0°C for 4 hours, after post-treatment and purification, 5-[(ethoxycarbonyl)methyl]-2-bromophenyl trifluoromethanesulfonate was obtained, shallow Yellow solid (3.4 g), yield 79%.
[0066] B) Preparation of ethyl 2-{4-bromo-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}acetate:
[0067] 5-[(Ethoxycarbonyl)methyl]-2-bromophenyl triflate (3.2g, 8.2mmol) was dissolved in toluene (50mL), and 4-(4-piperidinyl)morpholine was added (2.6g, 15.3mmol), sodium ethoxide (1.2g, 17.6mmol), the reaction mixture was stirred at 90°C for 18 hours, the reaction solution was cooled to room temperature, water (30mL) was added, cooled to 0°C and crystallized for 5 hours, filtered to obtain Ethyl 2-{4-b...
Embodiment 3
[0081] A) Preparation of 5-[(ethoxycarbonyl)methyl]-2-bromophenyl triflate:
[0082] 2-(4-Bromo-3-hydroxyphenyl)ethyl acetate (2.5g, 9.2mmol) was dissolved in pyridine (1.8g, 22.8mmol), slowly added dropwise trifluoromethanesulfonic anhydride (3.8g, 13.5mmol) , stirred at 25°C for 1 hour, after post-treatment and purification, 5-[(ethoxycarbonyl)methyl]-2-bromophenyl trifluoromethanesulfonate was obtained as a pale yellow solid (3.5g), yield 98%.
[0083] B) Preparation of ethyl 2-{4-bromo-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}acetate:
[0084] 5-[(ethoxycarbonyl)methyl]-2-bromophenyl trifluoromethanesulfonate (3.5g, 9.0mmol) was dissolved in 1,4-dioxane (80mL), and 4-(4 -piperidinyl)morpholine (4.1g, 24.1mmol), sodium isopropoxide (2.2g, 26.8mmol), the reaction mixture was stirred and reacted at 110°C for 6 hours, the reaction solution was cooled to room temperature, added water (55mL), and cooled to Crystallized at 0°C for 6 hours, filtered to give ethyl 2-{4-bromo-3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com