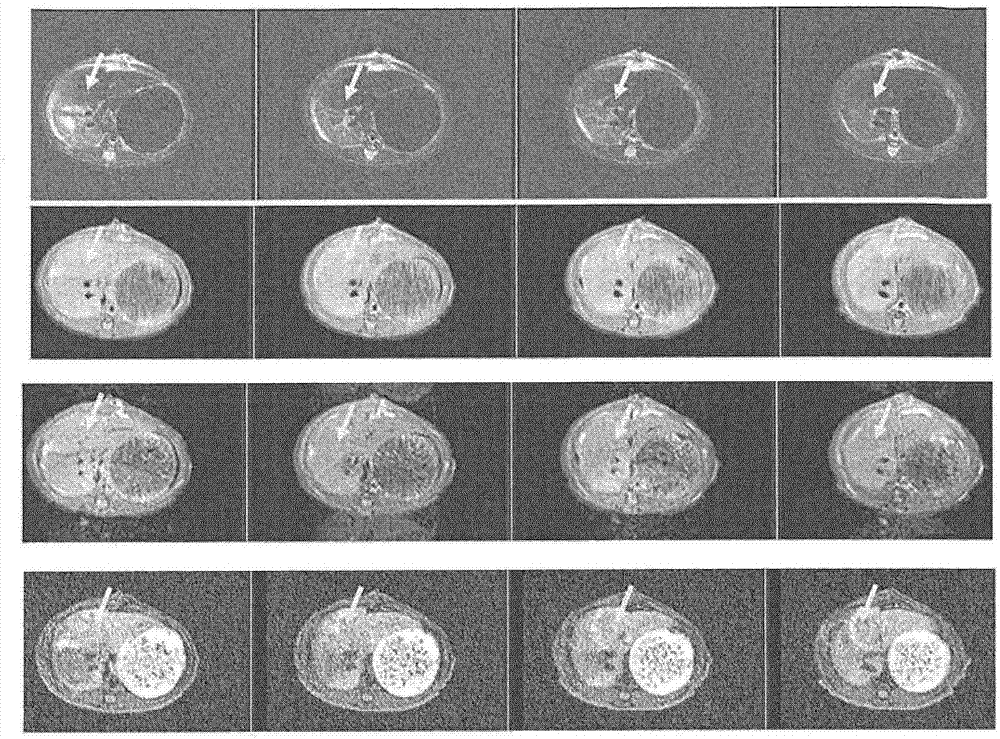
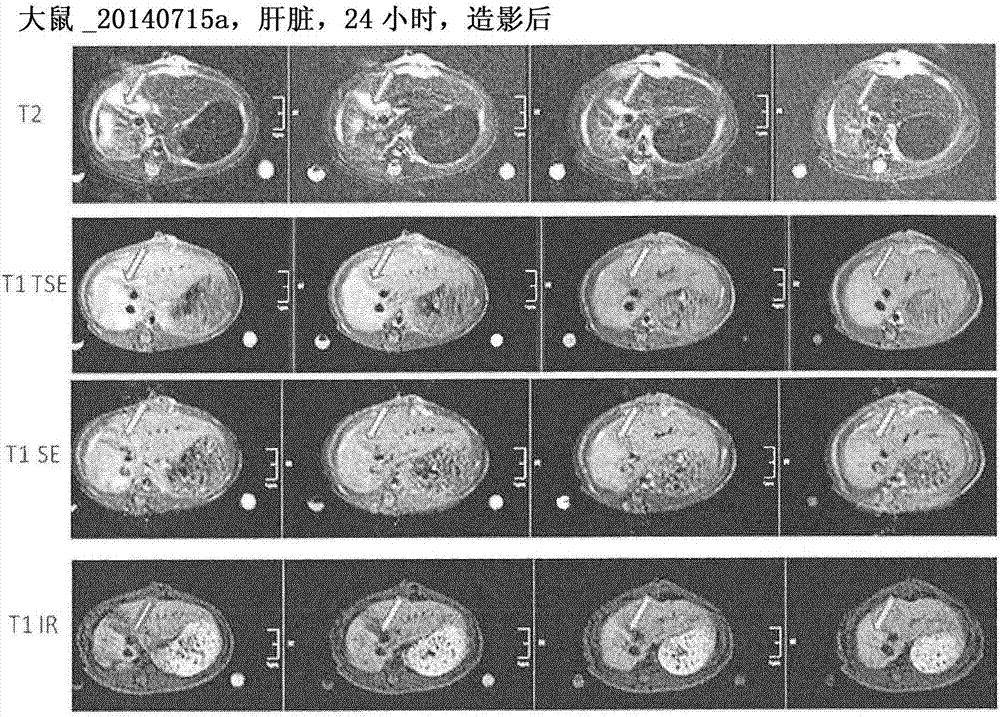
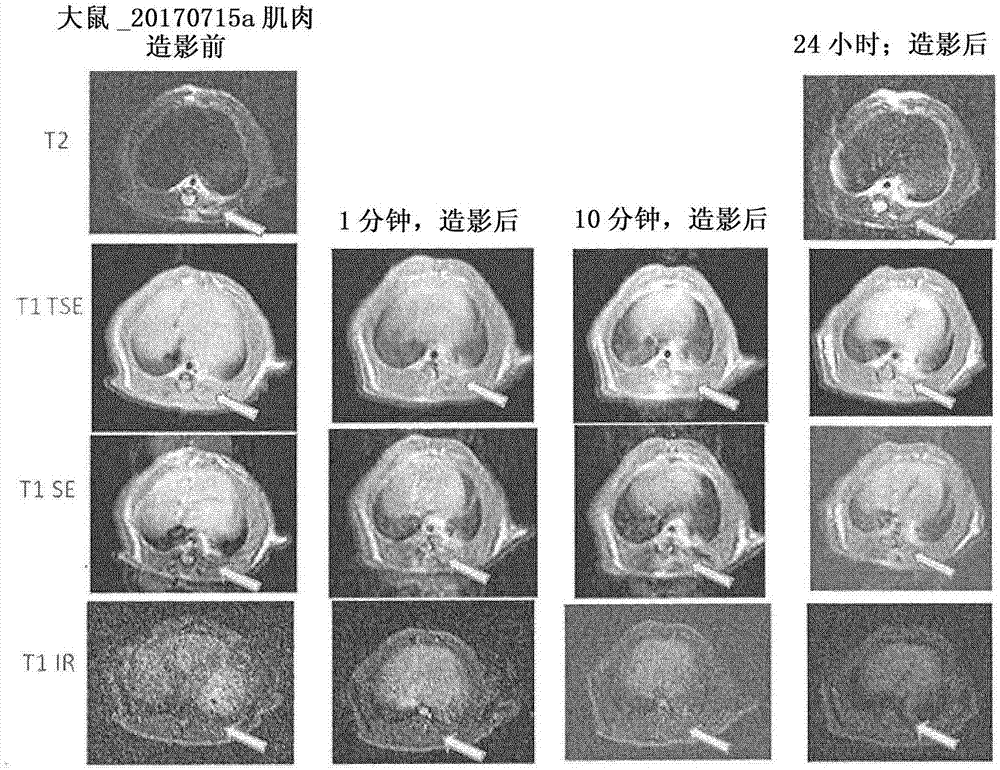
Targeted contrast agents comprising hydrazide functional group
A contrast agent and functional group technology, applied in the field of targeted contrast agents containing hydrazide functional groups, can solve problems such as toxicity, instability, and slow clearance rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Preparation of RF1311: The preparation included the steps in Scheme 1.
[0104] plan 1
[0105]
[0106] Compound 2. Under nitrogen atmosphere, ethyl indole-2-carboxylate (18.9 g, 100 mmol) was dissolved in 200 ml of ethanol; benzaldehyde (5.3 g, 50 mmol) was added, and the mixture was heated to reflux temperature. Concentrated HCl (3.7ml) was added and the reaction was left for 2 hours. After cooling, the white product was filtered off and washed well with cold ethanol. After the reaction can be TLC (CHCl 3 :hexane=1:1). The yield was 90%.
[0107] Compound 3. Compound 3 was prepared according to the modified procedure of Cresens, Erwin et al. (PCT / BE01 / 00192). 5.0 g (10.7 mmol) of compound 2 and 10 g of hydrazine monohydrate were dissolved in a mixture of 60 ml of pyridine and 30 ml of methanol. After the mixture was refluxed for 48 hours, the solvent was removed under reduced pressure. By adding H 2 O, H 2 O / methanol followed by addition of acetonitrile t...
Embodiment 2
[0111] Preparation of Rf1401: The preparation included the steps in Scheme 2.
[0112] Scenario 2
[0113]
[0114] Compound 2: Compound 1 (0.876g, 2mmol), DOTA-NHS-ester (1.0g, 2mmol, was prepared according to the procedure of Li, C.; Wong and W.-T. Tetrahedron, 2004, 60, 5595–5560 ) and DIPEA (0.284 g, 2.2 mmol) were dissolved in dry DMF (40 mL), and the resulting mixture was stirred at room temperature for 24 hours. After adding water, the solvent was removed under reduced pressure, and the resulting white powder was dissolved in in acetonitrile / H 2 O (1:1, v / v) and purified by preparative RP-HPLC. Yield: 0.59 g of white solid (0.72 mmol; 36%). 1H NMR (D 2 O)d 7.6-6.6, 14H, aromatic hydrogen and -CH; 3.7-3.0, 24H, methylene hydrogen.
[0115] RF1401: Compound 2 (0.5 mmol) was dissolved in water (30 ml), and gadolinium(III) acetate (0.5 mmol) was added slowly. During the addition, the pH was maintained at 7.4 with sodium hydroxide. After the addition, the mixture w...
Embodiment 3
[0117] RF1402 was prepared as a control compound.
[0118] Preparation of Rf1402: The preparation included the steps in Scheme 3.
[0119] Option 3
[0120]
[0121]Compound 2: DOTA (2.2 g, 5 mmol) was dissolved in 100 ml of distilled water, and the pH was adjusted to 4.8 with NaOH. The solution was cooled to 4°C and stirred. N-Ethyl-N'-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI, 1.92 g 10 mmol) was added followed by p-aminoaniline (7 mmol). The mixture was stirred at 4°C for 1 hour, then at room temperature for 24 hours. Purification was performed by using a preparative C18 column (200 g). The column was eluted with 10% methanol in water. The pure fractions were combined and evaporated to dryness to give the desired product as a white solid.
[0122] RF1402: Compound 2 (1.0 mmol) was dissolved in water (60 ml), and gadolinium(III) acetate (1.0 mmol) was added slowly. During the addition, the pH was maintained at 7.4 with sodium hydroxide. After the add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum tolerated dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com