Method for preparing hexanedioic acid from furan-2,5-dicarboxylic acid
A technology of dicarboxylic acid and adipic acid is applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc., to achieve the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] Hydrogenation catalyst 2%Ru / TiO 2 Preparation of:
[0064] 0.1mol / L of RuCl 2 Solution 2.1mL and 3.0mL deionized water were mixed, stirred evenly, and then the TiO 2 1.00 g of the carrier was added to the mixture, stirred and impregnated at room temperature for 10 hours, evaporated to dryness, and then dried in an oven at 110° C. for 12 hours to obtain a catalyst precursor. The loading amount of Ru is 2% (mass percentage). The precursor prepared in the above steps was placed in a quartz tube, first calcined at 500 ° C in air for 4 h, and then in 20% H 2 +N 2 Reduction at a temperature of 200°C for 3 hours to obtain a supported 2% Ru / TiO 2 catalyst.
[0065] Prepare 2% Rh / ZrO as above 2 and 2%Pt / TiO 2 .
[0066] Hydrodeoxygenation catalyst 2%Ir / 40%WO 3 / TiO 2 (co-loaded) preparation:
[0067] Mix 0.76g ammonium metatungstate with 5.0mL water, stir evenly, and then put TiO 2 1.00 g of the carrier was added to the mixture, stirred and impregnated at room tempe...
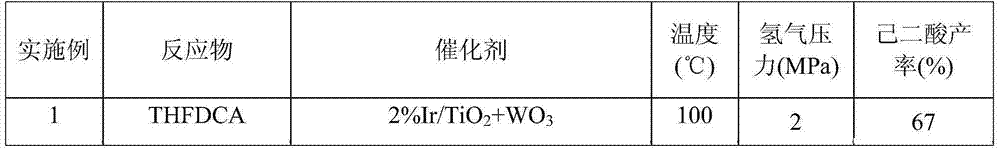
Embodiment 1
[0071] The preparation of embodiment 1, adipic acid
[0072] 1. Preparation of tetrahydrofuran-2,5-dicarboxylic acid (THFDCA)
[0073] In a 30mL autoclave, add 0.2g of the 2% Ru / TiO prepared above 2 Catalyst, 1g FDCA and 10mL water (the mass percentage composition of FDCA is 10%), after the reactor is airtight, fill the residual air in the 2MPa hydrogen replacement reactor, after repeating three times, charge 4MPa hydrogen in the reactor, put The reaction kettle was placed on a heating furnace and heated to a reaction temperature of 120° C., and stirred and reacted at a rotation speed of 700 rpm for 6 hours. After the reaction, take out the reaction kettle from the heating furnace, cool to room temperature, reduce the pressure in the kettle to normal pressure, open the lid of the kettle, take out the liquid-solid mixture and separate it by suction filtration, set the volume of the obtained liquid to 50mL, and use high-efficiency Analysis was performed by liquid chromatograph...
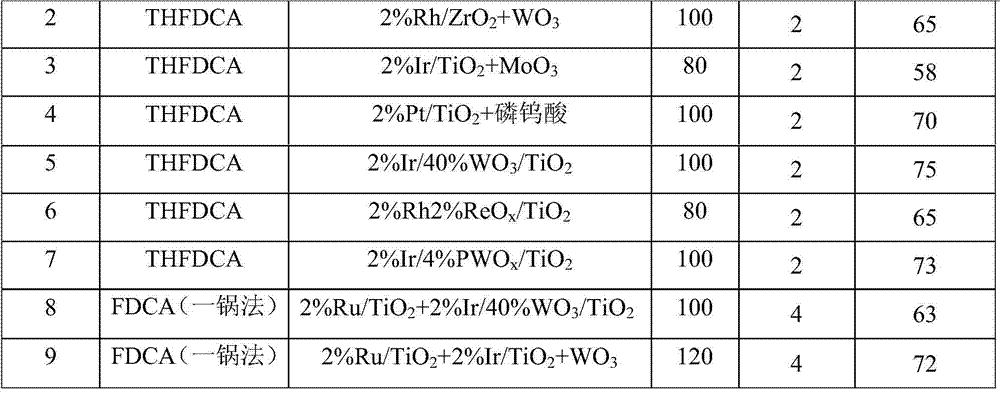
Embodiment 2
[0077] Embodiment 2, prepare adipic acid by tetrahydrofuran-2,5-dicarboxylic acid (THFDCA)
[0078] With 2%Rh / ZrO 2 +WO 3 as a catalyst.
[0079] In a 30mL autoclave, add 0.2g2%Rh / ZrO 2 +WO 3 Catalyst (wherein the molar ratio of Rh and THFDCA is about 1:30) and 2% THFDCA aqueous solution prepared in 10mL embodiment 1, after the reactor is airtight, fill the residual air in 2MPa hydrogen replacement reactor, after repeating three times, to reaction The kettle was filled with 2MPa hydrogen gas, the reaction kettle was placed on a heating furnace to be heated to a reaction temperature of 100° C., and the reaction was stirred and reacted at a rotation speed of 700 rpm for 20 hours. After the reaction finished, take out the reaction kettle from the heating furnace, cool to room temperature, reduce the pressure in the kettle to normal pressure, open the lid, take out the liquid-solid mixture and separate it by suction filtration, and analyze the liquid obtained by liquid chromat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com