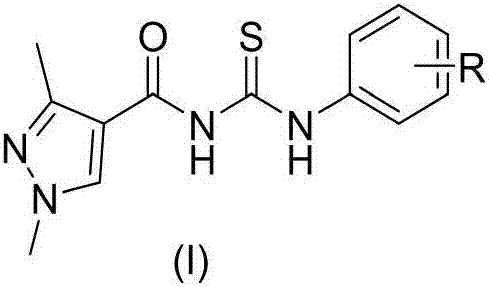
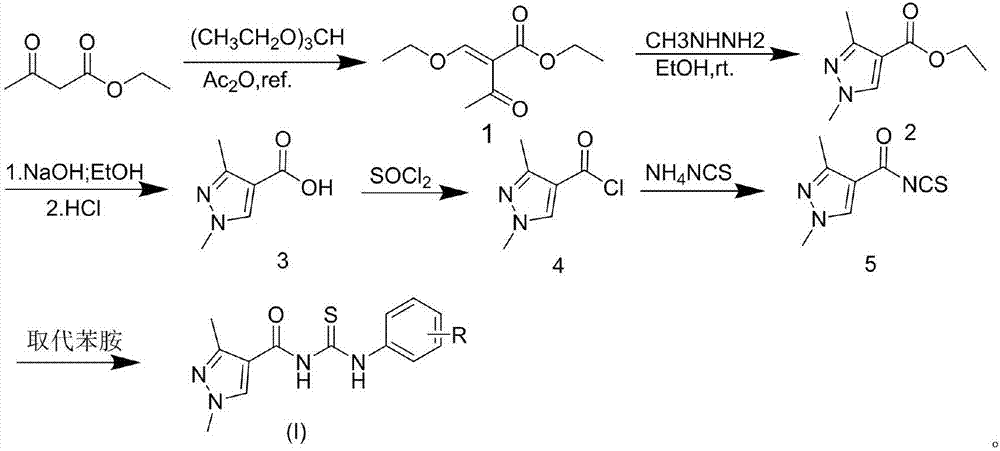
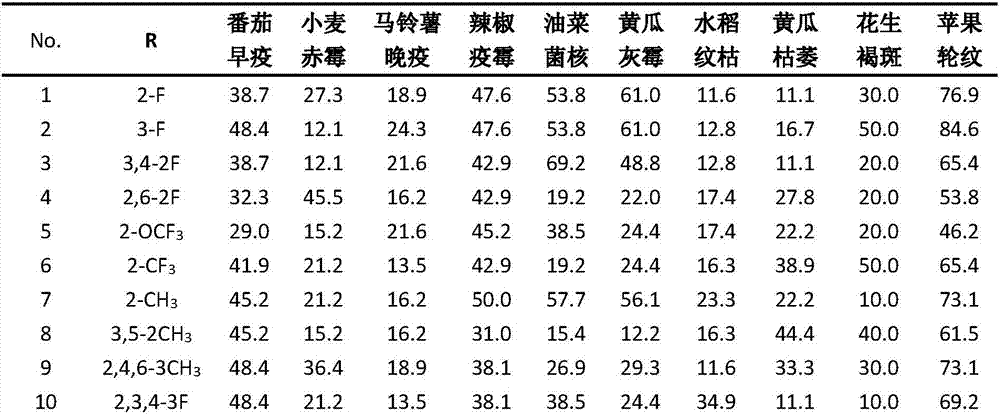
Acyl thiourea compound containing 1,3-dimethyl-1H-pyrazole structure and preparation method and application thereof
A technology for acylthiourea compounds, which is applied in the field of preparation of acylthiourea compounds, can solve problems such as molecular structure changes, and achieve the effects of convenient post-processing, simple preparation methods, and high product yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] N-((2-fluorophenyl)-1,3-dimethyl-1H-pyrazole-4-ylthiourea, white crystal, yield 54.4%, m.p.222-224℃; 1 H NMR (CDCl 3 ,500MHz),:11.46(s,1H,-NH-), 9.52(s,1H,Ar-NH-),8.28(s,1H,pyrazole H),8.09-8.00(m,1H,Ar-H) , 7.17-7.11(m,1H,Ar-H),6.81(s,1H,Ar-H),3.92(s,3H,N-CH 3 ),2.57(s,3H,pyrazole CH 3 ); HRMS calcd for C 13 h 13 FN 4 OS 293.0867, found 293.0860 [M+1] + .
Embodiment 2
[0033] N-((3-fluorophenyl)-1,3-dimethyl-1H-pyrazole-4-ylthiourea, white crystal, yield 30.7%, m.p.186-188℃; 1 H NMR (CDCl 3 ,500MHz),δ:11.34(s,1H,-NH-), 10.02(s,1H,Ar-NH-),8.27(s,1H,pyrazole H),7.90(s,1H,Ar-H), 7.74 (d, J = 8.29Hz, 1H, Ar-H), 7.49 (t, J = 7.87, 7.87Hz, 1H, Ar-H), 7.42 (d, J = 7.76 Hz, 1H, Ar-H), 3.73(s,3H,N-CH 3 ),2.53(s,3H,pyrazole CH 3 ); HRMS calcd for C 13 h 13 FN 4 OS 293.0867, found 293.0891[M+1] + .
Embodiment 3
[0035]N-((3,4-difluorophenyl)-1,3-dimethyl-1H-pyrazole-4-ylthiourea, light yellow solid, yield 40.9%, m.p.201-203℃; 1 H NMR (CDCl 3 ,500MHz),δ:11.26(s, 1H,-NH-),10.18(s,1H,Ar-NH-),8.38(s,1H,pyrazole H),7.76(s,1H,Ar-H), 7.32-7.27(m, 2H, Ar-H), 7.14(d, J=7.43Hz, 1H, Ar-H), 3.81(s, 3H, N-CH 3 ), 2.52(s,3H,pyrazole CH 3 ); HRMS calcd for C 13 h 12 f 2 N 4 OS 311.0773, found 311.0768[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com