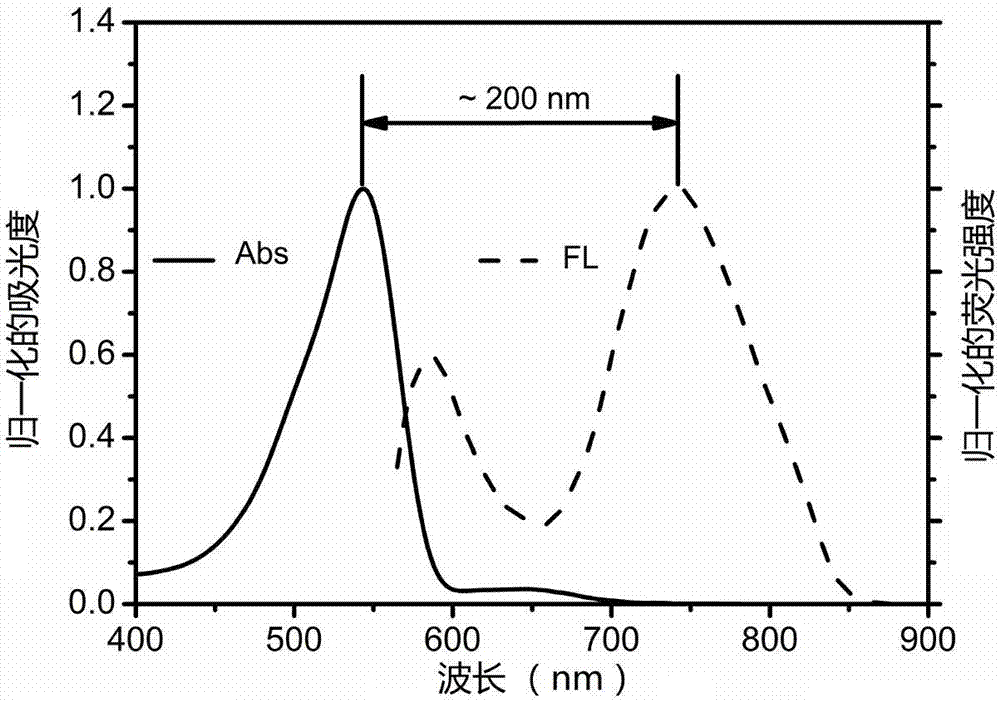
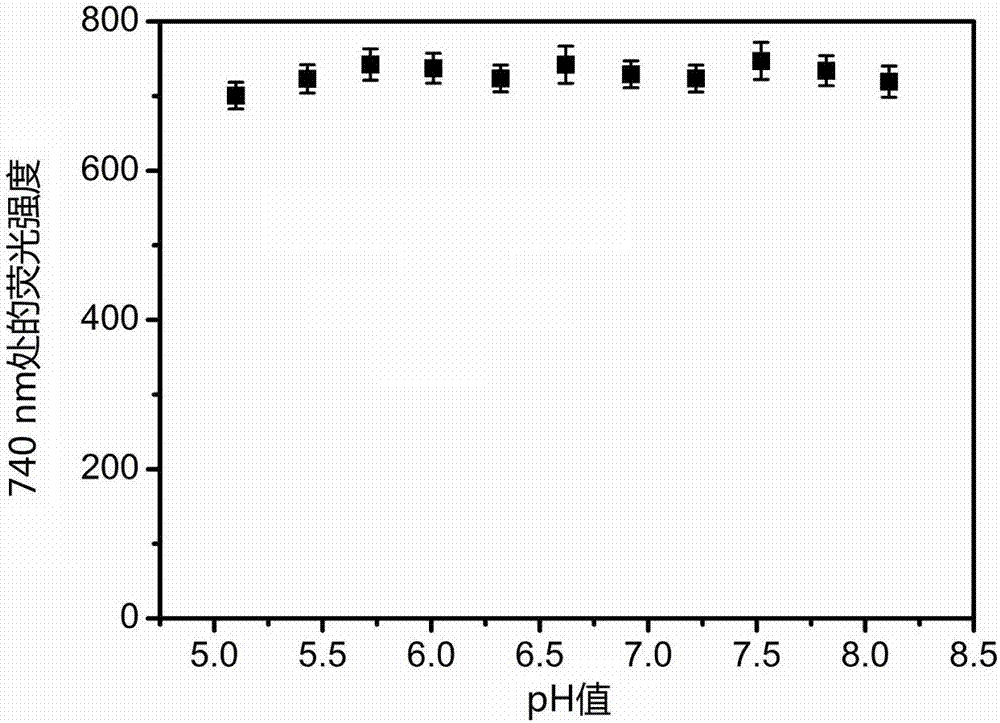
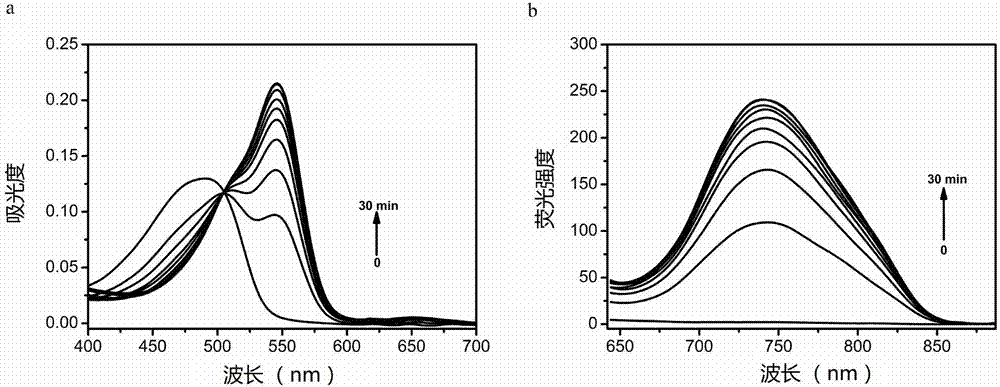
Large-Stokes-displacement near-infrared BODIPY (boron-dipyrromethene) dye and preparation and application thereof
A near-infrared and dye technology, applied to organic dyes, azo dyes, analytical materials, etc., can solve the problems of difficult synthesis and limited application, and achieve the effects of reduced detection sensitivity, reduced fluorescence self-quenching, and reduced photodamage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The synthesis of embodiment 1 compound NIR-BOD (X=S, O)
[0040] N 2 Under gas protection, HO-B-CHO (130mg, 0.3mmol; R 1 , R 2 , R 4 =CH 3 , R 3 =CH 3 CH 2 ), o-aminothiophenol (75.1mg, 0.6mmol) and sodium metabisulfite (57.7mg, 0.304mmol) were refluxed for 2 hours, cooled, spin-dried the solvent, and purified by column chromatography to obtain a reddish-brown solid with a yield of 52.8%. When X=0, it is consistent with the method in Example 1. (HO-B-CHO is self-made in this laboratory, the self-made method refers to the patent number: ZL 201310636785.7, and o-aminothiophenol and sodium metabisulfite are purchased)
Embodiment 2
[0041] The synthesis of embodiment 2 compound NIR-BOD (X=N)
[0042] N 2 Under gas protection, HO-B-CHO (200mg, 0.46mmol; R 1 , R 2 , R 4 =CH 3 , R 3 =CH 3 CH 2 ), o-phenylenediamine (99.5mg, 0.92mmol) was refluxed for 2 hours, cooled, spin-dried to dry the solvent, and purified by column chromatography to obtain a dark brown solid with a yield of 43.2%.
Embodiment 3
[0043] The synthesis of embodiment 3 compound BOD-thiol (X=S)
[0044] Dissolve compound NIR-BOD (50mg, 0.093mmol, R 1 , R 2 , R 4 =CH 3 , R 3 =CH 3 CH 2 ), and triethylamine (25.8 μL, 0.19 mmol) was added thereto, and then 2,4-dinitrobenzenesulfonyl chloride (50.7 mg, 0.19 mmol) was added under ice bath, then the ice bath was removed, and the reaction was carried out at room temperature for 1 h . After the reaction was completed, the solvent was distilled off and separated and purified by column chromatography to obtain a red solid with a yield of 39.0%. (X=O, the method in the preparation method example 3 of N is consistent)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com