Application of lycorine hydrochloride in treatment of pulmonary fibrosis
A technology of lycorine hydrochloride and pulmonary fibrosis, which is applied in the directions of medical preparations containing active ingredients, respiratory diseases, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
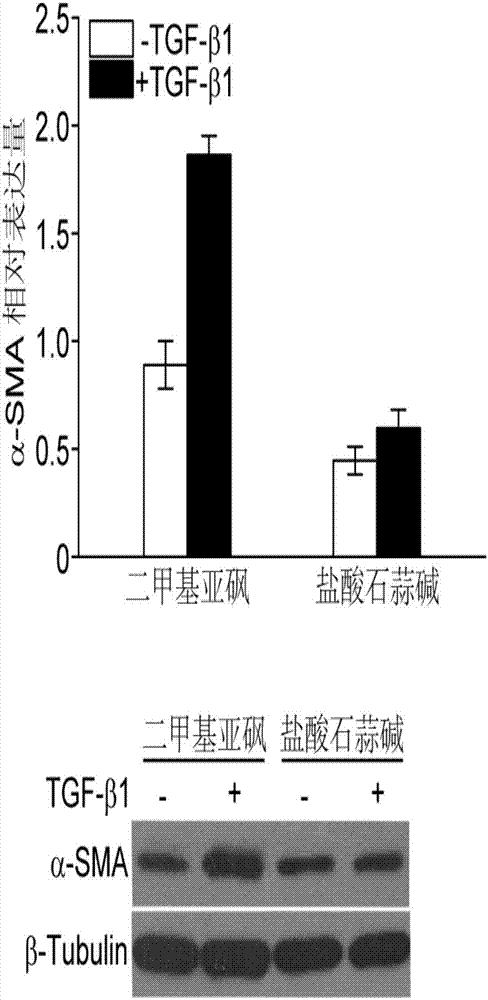
[0015] Example 1 Lycorine Hydrochloride Inhibits Myofibroblast Differentiation Induced by TGF-β1
[0016] The cells used in this example were the mouse lung fibroblast cell line Mlg obtained from the American Type Culture Collection (ATCC). After Mlg fibroblasts were overgrown, 0.1% serum was treated for 24 hours, and 5 ng / mL LTGF-β1 protein and a final concentration of 5 μmol / L lycorine hydrochloride or the same volume of DMSO were added as control. After 12 hours of treatment, RNA was collected for real-time fluorescent quantitative PCR to detect α-SMA; after 24 hours of treatment, cells were collected and Western Blotting was used to detect the expression of α-SMA protein. Real-time fluorescent quantitative PCR refers to preparing total RNA from cells using Trizol (Invitrogen), synthesizing cDNA using ThermoScript RT-PCR kit (Invitrogen), and detecting the expression of α-SMA using SYBR Green kit (Roche). Relative gene expression using 2 -Δ(ΔCT) method, and β-actin was us...
Embodiment 2
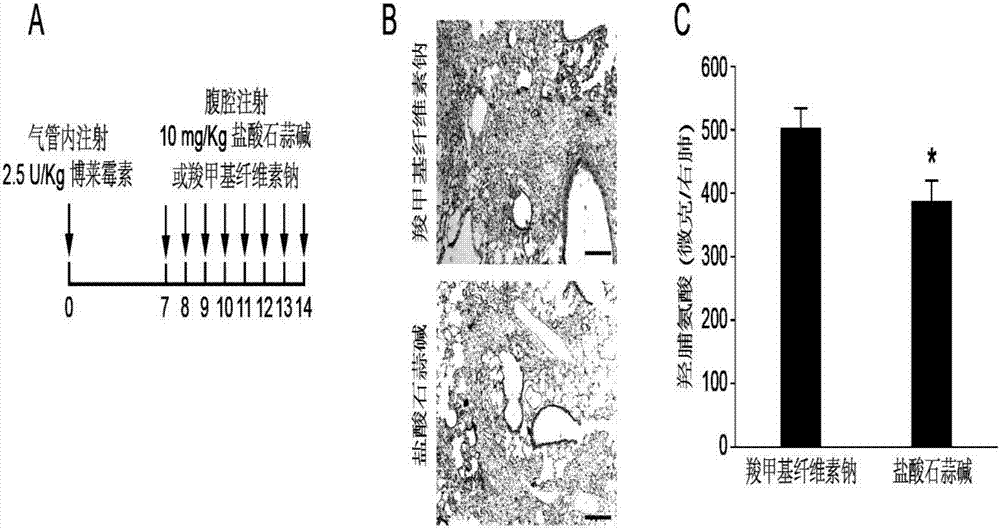
[0023] Example 2 Lycorine hydrochloride slows down bleomycin-induced pulmonary fibrosis
[0024] Pulmonary fibrosis animal model preparation refers to male C57BL / 6J (age 8-10 weeks) wild-type mice, anesthetized mice with 0.6mL / 100g intraperitoneal (I.P.) injection of 7.5% chloral hydrate, intratracheal injection of Bray Mycin 2.5U / kg. The specific scheme is as follows: after weighing and recording the body weight, fix the mouse on the operating table, disinfect the neck with 70% alcohol, use a scalpel to cut vertically about 1 cm in the neck of the mouse, use micro-tweezers to separate the tissue to expose the trachea, Insert the syringe into the trachea through the gap between the cartilage rings of the trachea towards the end of the heart, then slowly inject 2.5 U / kg of bleomycin physiological saline solution in a volume appropriate to its body weight, and immediately turn the animal upright and rotate left and right to make the liquid medicine Evenly distributed in the lun...
Embodiment 3
[0032] Preparations of lycorine hydrochloride
[0033] Lycorine Hydrochloride Tablets
[0034] Lycorine hydrochloride 5mg, starch 92g, magnesium stearate 3g
[0035] Preparation process: take lycorine hydrochloride and pass through a 100-mesh sieve, add starch and magnesium stearate, mix evenly, make granules, dry, and press into tablets.
[0036] Lycorine Hydrochloride Capsules
[0037] Lycorine hydrochloride 5mg, pregelatinized starch 92g, magnesium stearate 3g
[0038] Preparation process: take lycorine hydrochloride and pass through a 100-mesh sieve, add pregelatinized starch and magnesium stearate, mix evenly, make granules, dry, pack into capsules, and obtain.
[0039] Lycorine Hydrochloride Injection
[0040] Lycorine hydrochloride 43mg, sodium chloride for injection 7mg
[0041] Preparation process: take lycorine hydrochloride through a 100-mesh sieve, add 1.9mL of water for injection to dissolve, add sodium chloride for injection to make it isotonic, adjust the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com