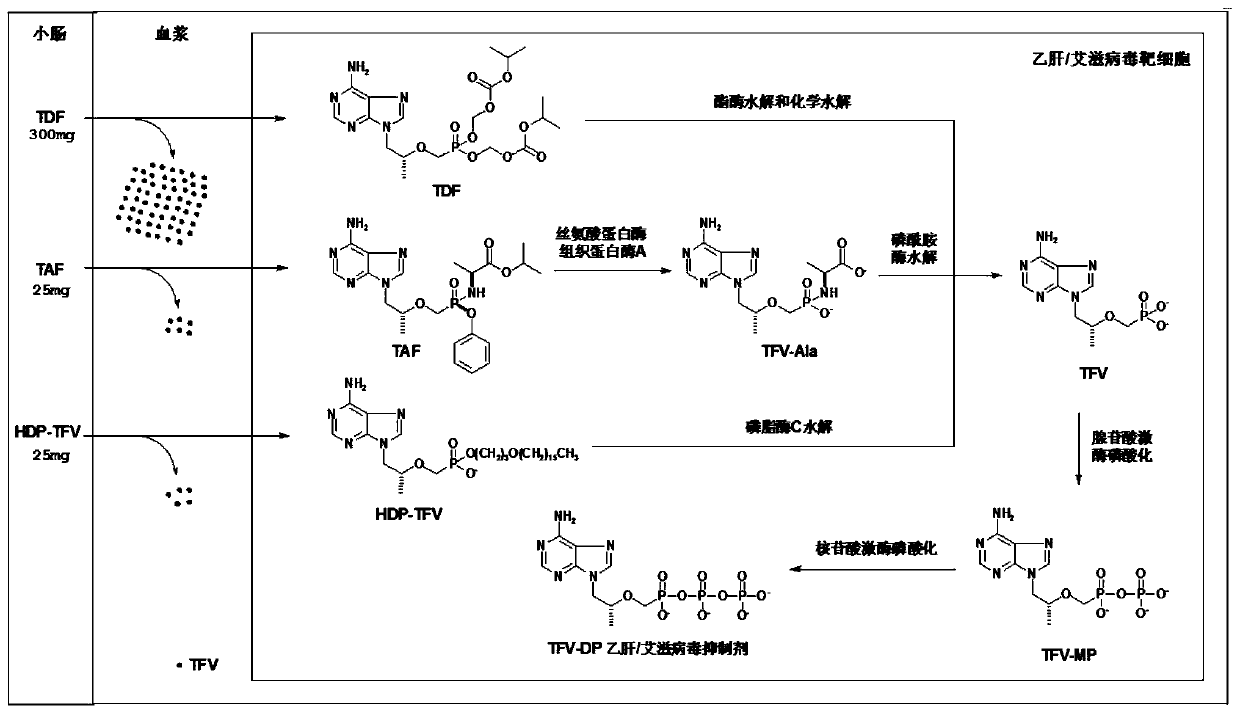
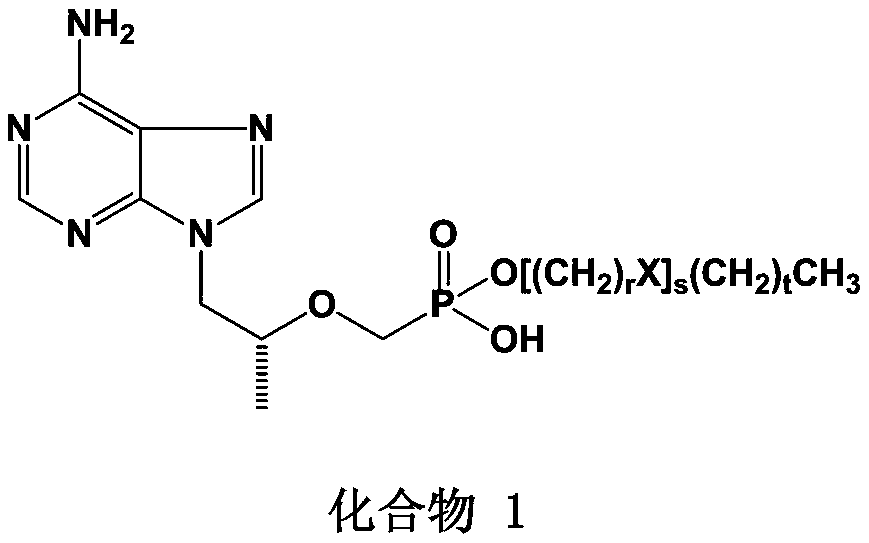
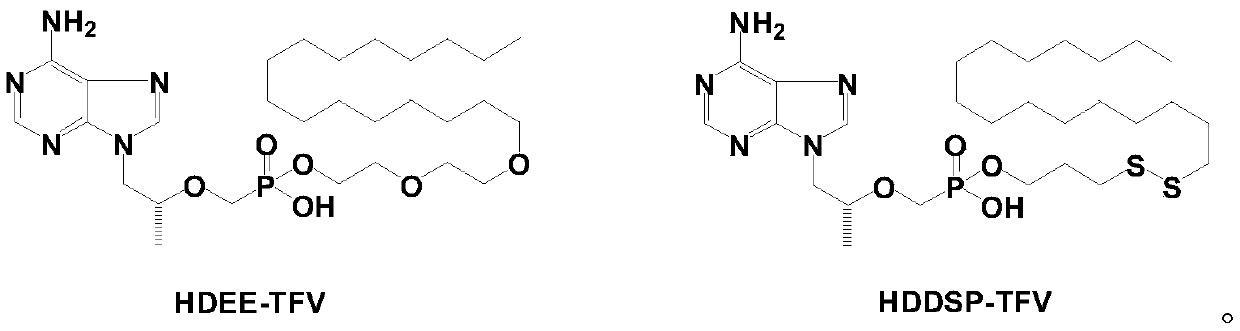
Tenofovir lipid monoester compound as well as preparation method and application thereof
A technology of tenofovir, compound, applied in the field of chemical pharmacy, can solve the problems of nephrotoxicity and poor oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1 Preparation of tenofovir mono(hexadecyloxyethoxyethyl) ester (HDEE-TFV)
[0110]
[0111] 1) Slowly add 120 ml of oxalyl chloride dropwise to 100 g of tenofovir (TFV), 50 g of N,N-dimethylformamide and 1 liter of dichloromethane under nitrogen protection at 15-25°C with stirring. In the mixture composed of methane, stir and reflux for 4 to 6 hours, then cool to room temperature; concentrate under reduced pressure at 50°C to remove volatiles, add 1 liter of dichloromethane to dissolve the residue for later use;
[0112] 2) Under nitrogen protection, 0 ~ 10 ° C, under stirring, slowly drop the mixture of 125 grams of hexadecyloxyethoxyethanol (HDEE), 200 milliliters of triethylamine and 500 milliliters of dichloromethane to step 1 ) into the dichloromethane solution obtained, stirred at room temperature for 4 hours, concentrated under reduced pressure at 50° C. to remove volatile matter; added 500 milliliters of ethyl acetate, stirred at room temperature to...
Embodiment 2
[0118] Example 2 Preparation of tenofovir mono(hexadecyloxyethoxyethyl) ester (HDEE-TFV)
[0119]
[0120] 1) Under anhydrous and anaerobic conditions, 100 grams of tenofovir (TFV), 125 grams of hexadecyloxyethoxyethanol (HDEE), 110 grams of N,N,-dicyclohexylcarbodiimide and The mixture composed of 600 ml of pyridine was stirred at 90°C for 18 hours, then cooled to room temperature; filtered, the filter cake was rinsed with dichloromethane, and the combined filtrate was concentrated under reduced pressure at 60°C to remove volatiles;
[0121] 2) Add 500 ml of ethyl acetate, stir at room temperature to disperse evenly, add 500 ml of 3N hydrochloric acid, and continue stirring for 30 minutes;
[0122] 3) Static layering, after the aqueous layer was extracted with 200 ml of ethyl acetate, the pH was adjusted to 3 with 35% sodium hydroxide; the aqueous phase was extracted with dichloromethane (500 ml x 2); the dichloromethane was distilled off under reduced pressure , add 50...
Embodiment 3
[0125] Example 3 Preparation of tenofovir mono(hexadecyloxyethoxyethyl) ester (HDEE-TFV)
[0126]
[0127] 1) Stir a mixture of 100 grams of tenofovir (TFV), 20 grams of sodium hydroxide and 500 milliliters of N,N-dimethylformamide at room temperature for 3 hours, then concentrate under reduced pressure at 80°C to remove volatiles ; Add 500 milliliters of N,N-dimethylformamide and 150 grams of hexadecyloxyethoxyethanol methanesulfonate (HDEEMs), under nitrogen protection, stir at 100 ° C for 18 hours, then cool to room temperature; , concentrated under reduced pressure at 80°C to remove volatiles;
[0128] 2) Add 500 ml of ethyl acetate, stir at room temperature to disperse evenly, add 500 ml of 3N hydrochloric acid, and continue stirring for 30 minutes;
[0129] 3) Static layering, after the aqueous layer was extracted with 200 ml of ethyl acetate, the pH was adjusted to 3 with 35% sodium hydroxide; the aqueous phase was extracted with dichloromethane (500 ml x 2); the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com