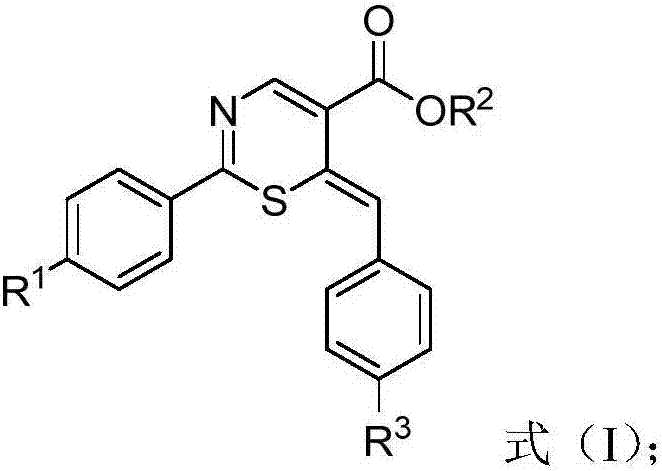
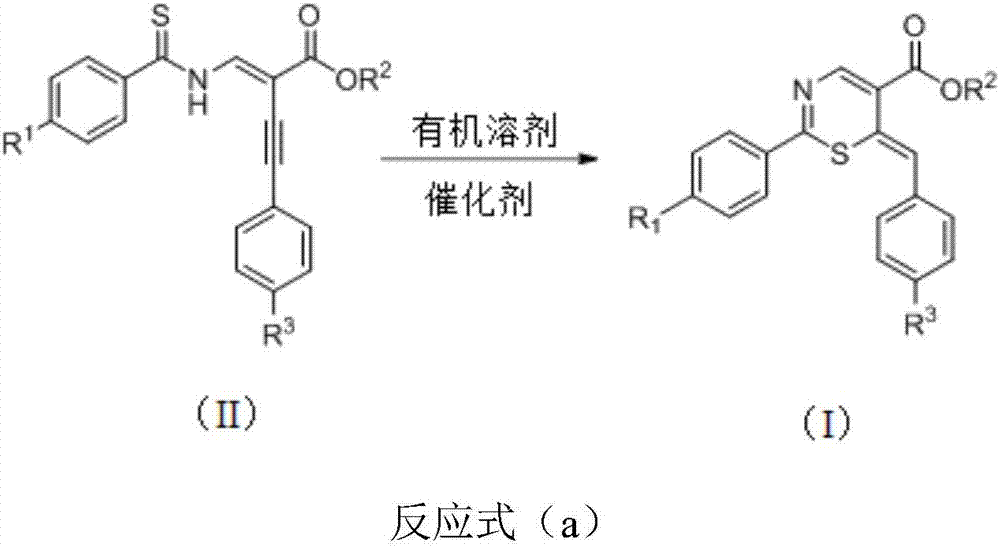
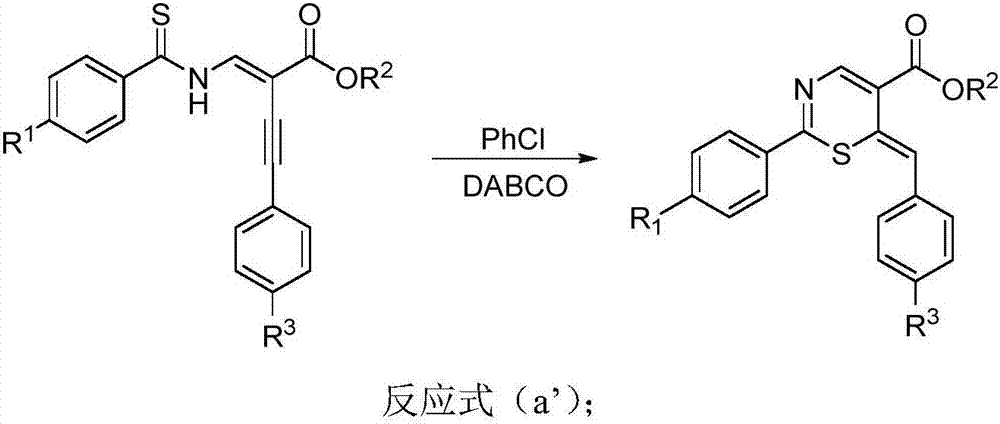
Thiazine derivative and synthetic method thereof
A synthesis method and derivative technology, applied in the direction of organic chemistry, can solve environmental pollution and other problems, and achieve the effects of environmental friendliness, cheap catalyst, and convenient post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Synthesis of cis-6-benzylidene-2-phenyl-6H-1,3-thiazine-5-carboxylic acid ethyl ester
[0030]
[0031] Thiobenzamido-substituted enynyl esters, DABCO, and solvents are selected respectively: trans-4-phenyl-2-(phenylthioiminomethylene) but-3-ynoic acid ethyl ester, DABCO, chlorine Benzene, the amount of raw materials used is trans-4-phenyl-2-(phenylthioiminomethylene) but-3-ynoic acid ethyl ester 0.2mmol, DABCO 1mmol, chlorobenzene 2ml. React at 110°C for 22 hours to obtain the target product as a brown liquid with an isolated yield of 90%.
[0032] NMR data: 1 H NMR (400MHz, CDCl 3 )δ1.39(t, J=7.2Hz, 3H), 4.36(q, J=6.8Hz, 2H), 7.26-7.34(m, 2H), 7.36-7.47(m, 6H), 7.49-7.56(m ,1H),7.95(d,J=7.6Hz 2H),8.00(s,1H); 13 C NMR (100MHz, CDCl 3 )δ13.94, 61.29, 114.74, 119.89, 127.72, 128.26, 128.39, 128.90, 129.43, 129.84, 132.87, 135.68, 136.79, 145.04, 166.20, 166.69;
[0033] High-resolution mass spectrometry data: HRMS(EI)calcd for C 20 h 17 NO 2 S: 335...
Embodiment 2
[0034] Example 2: Synthesis of cis-6-benzylidene-2-phenyl-6H-1,3-thiazine-5-carboxylic acid ethyl ester
[0035]
[0036] Thiobenzamido-substituted enynyl esters, K 2 CO 3 , The solvent is selected respectively: trans-4-phenyl-2-(phenylthioiminomethylene) but-3-ynoic acid ethyl ester, K 2 CO 3 , Chlorobenzene, raw material consumption is trans-4-phenyl-2-(phenylthioiminomethylene) but-3-ynoic acid ethyl ester 0.2mmol, K 2 CO 3 2.0mmol, chlorobenzene 2ml. React at 110°C for 24 hours to obtain the target product as a brown liquid with an isolated yield of 50%.
[0037] NMR data: 1 H NMR (400MHz, CDCl 3 )δ1.39(t, J=7.2Hz, 3H), 4.36(q, J=6.8Hz, 2H), 7.26-7.34(m, 2H), 7.36-7.47(m, 6H), 7.49-7.56(m ,1H),7.95(d,J=7.6Hz 2H),8.00(s,1H); 13 C NMR (100MHz, CDCl 3)δ13.94, 61.29, 114.74, 119.89, 127.72, 128.26, 128.39, 128.90, 129.43, 129.84, 132.87, 135.68, 136.79, 145.04, 166.20, 166.69;
[0038] High-resolution mass spectrometry data: HRMS(EI)calcd for C 20 h 17 NO 2 S:...
Embodiment 3
[0039] Example 3: Synthesis of cis-6-benzylidene-2-phenyl-6H-1,3-thiazine-5-carboxylic acid ethyl ester
[0040]
[0041] Thiobenzamido-substituted enynyl esters, DABCO, and solvents are selected respectively: trans-4-phenyl-2-(phenylthioiminomethylene) but-3-ynoic acid ethyl ester, DABCO, chlorine Benzene, the amount of raw materials used is trans-4-phenyl-2-(phenylthioiminomethylene) but-3-ynoic acid ethyl ester 0.2mmol, DABCO 0.04mmol, toluene 2ml. React at 80°C for 34 hours to obtain the target product as a brown liquid with an isolated yield of 75%.
[0042] NMR data: 1 H NMR (400MHz, CDCl 3 )δ1.39(t, J=7.2Hz, 3H), 4.36(q, J=6.8Hz, 2H), 7.26-7.34(m, 2H), 7.36-7.47(m, 6H), 7.49-7.56(m ,1H),7.95(d,J=7.6Hz 2H),8.00(s,1H); 13 C NMR (100MHz, CDCl 3 )δ13.94, 61.29, 114.74, 119.89, 127.72, 128.26, 128.39, 128.90, 129.43, 129.84, 132.87, 135.68, 136.79, 145.04, 166.20, 166.69;
[0043] High-resolution mass spectrometry data: HRMS(EI)calcd for C 20 h 17 NO 2 S: 335.098...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Separation yield | aaaaa | aaaaa |
| Separation yield | aaaaa | aaaaa |
| Separation yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com