Method for detecting circulating tumor cell in whole blood by utilizing ICP-MS and fluorescence imaging
An ICP-MS, fluorescence imaging technology, applied in the field of biological analysis, can solve the problems of magnetic particle loss, sensitivity reduction, signal fluctuation, etc., to eliminate errors, improve stability and reliability, strong simultaneous analysis and accurate quantification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment l
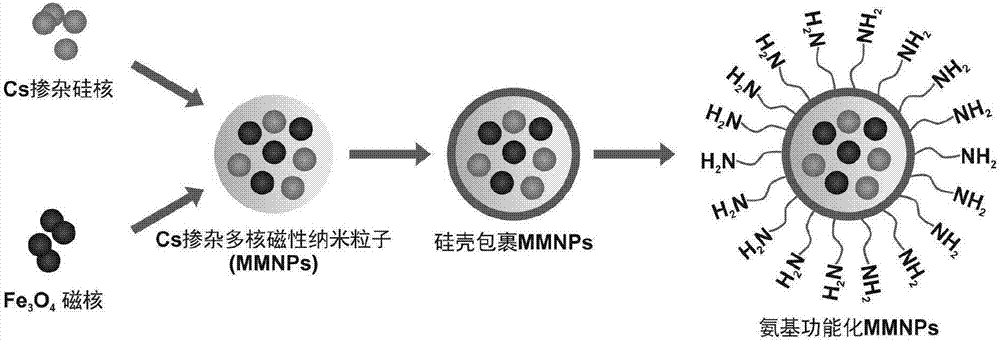
[0036] Example 1: Preparation of Amino-modified Cs-doped Multinuclear Magnetic Nanoparticles, MMNPs-anti-EpCAM Probe Magnetic Balls and QDs-anti-ASGPR Labeled Probes
[0037] Schematic diagram of the preparation process of amino-modified Cs-doped multinuclear magnetic nanoparticles. figure 1 As shown, the specific steps are as follows:
[0038] (1) Preparation of magnetic nanoparticles Fe by co-precipitation method 3 o 4 : First weigh 1.35g FeCl 3 ·6H 2 O, 0.5gFeCl 2 4H 2 O, add 25mL deionized water; after the solid is completely dissolved, mechanically stir in an argon atmosphere and heat to reflux at 80°C; when the color of the solution turns dark orange, increase the argon flow rate, and quickly add 12.5mL concentrated ammonia water , at this time, the color of the solution changed from orange to black; continue heating and stirring for 20min, and then cool to room temperature to obtain magnetic nanoparticles Fe3 o 4 , using the magnetic separation method to wash the...
Embodiment 2
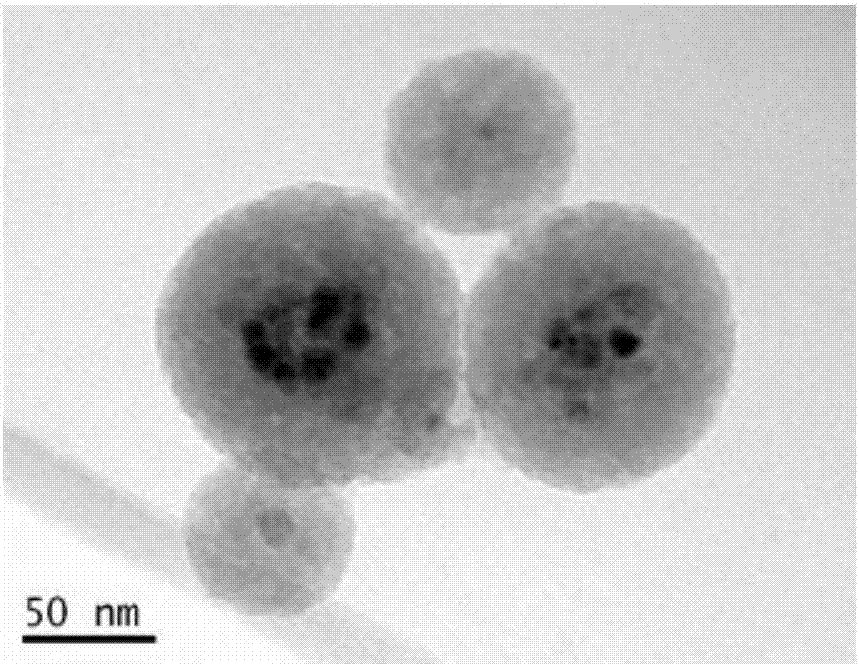
[0043] Example 2: Characterization of amino-modified Cs-doped multinuclear magnetic nanoparticles
[0044] Adopt transmission electron microscope (TEM) to observe the MMNPs-NH that embodiment 1 prepares 2 , its TEM picture is as figure 2 shown. from figure 2 It can be seen that MMNPs-NH 2 Good dispersion, relatively uniform particle size, about 100-150nm, it can be clearly observed that a magnetic ball contains multiple Fe 3 o 4 nuclear.
Embodiment 3
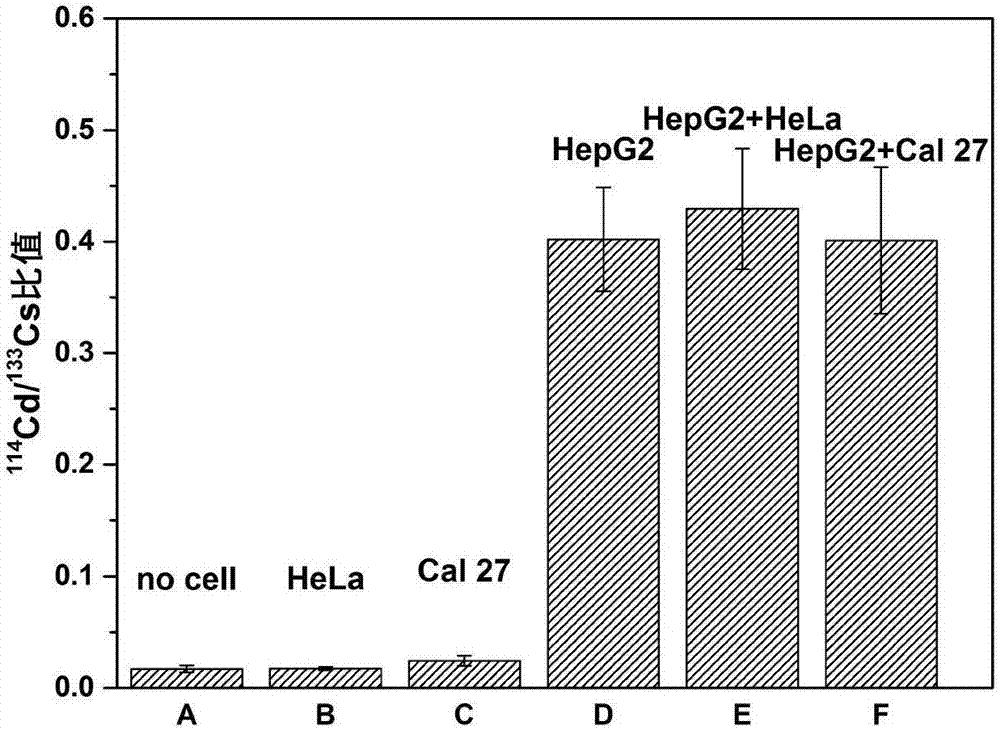
[0045] Example 3: ICP-MS analysis of target cells and non-target cells
[0046] In this example, cadmium selenide (CdSe) quantum dots were used as labeled quantum dots, HepG2 cells expressing ASGPR antigen (liver-specific transmembrane glycoprotein) were selected as target objects, and human tongue squamous cell carcinoma cells Cal 27 that did not express ASGPR were selected. cells and human cervical cancer HeLa cells as negative control cells. Group A contains HepG2 cells, group B contains 1×10 4 HeLa cells, group C contains 1×10 4 Cal-27 cells, group D contains 1×10 4 HepG2 cells, group E contained 1×10 4 HepG2 cells and 1×10 5 HeLa cells, group F contained 1×10 4 HepG2 cells and 1×10 5 Cal-27 cells.
[0047] Take 2 μL prepared MMNPs-anti-EpCAM probe magnetic balls, block with 200 μL bovine serum albumin solution (1% BSA) for 0.5 h, in order to reduce the non-specific adsorption of the probe; then add the cells to be tested and place on a shaker at 90 rpm Incubate at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com