Copper ion detection method based on click chemistry and detection kit
A technology of copper ion detection and click chemistry, which is applied in the field of environmental analytical chemistry, can solve the problems of expensive instruments and interference detection sensitivity, and achieve the effect of low cost, high sensitivity and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
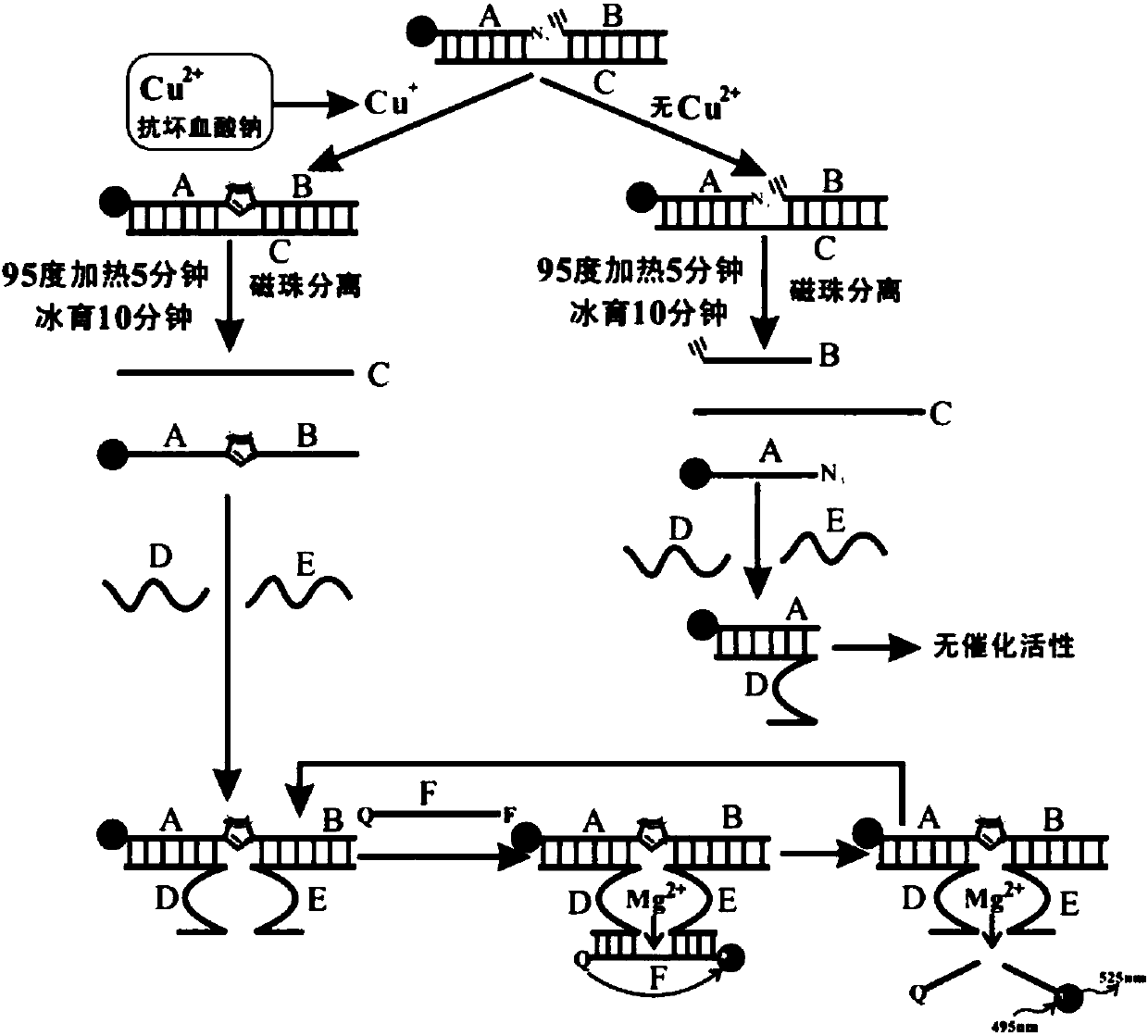
[0080] A method for detecting copper ions based on click chemistry is carried out as follows:
[0081] (1) Tris-HCl buffer (20 mM, pH 7.5, containing 100 mM NaCl and 15 mM MgCl 2 ) to dissolve nucleic acid sequences A, B, C, D, E, and F, respectively. 300 nM A, 300 nM B, and 500 nM C were mixed thoroughly at room temperature, and reacted for 30 minutes to form an A-B-C complex;
[0082] (2) Different concentrations of copper ions (Cu 2+ ) solution, and a five-fold concentration of sodium ascorbate solution were thoroughly mixed, added to the A-B-C complex, and reacted at room temperature for 60 minutes;
[0083] (3) Heat at 95°C for 5 minutes to separate C and A-B, and immediately incubate on ice for 10 minutes to keep C and A-B single-chain. Remove the supernatant after magnetic bead separation, and keep the A-B chain;
[0084] (4) Add 300 nM D and E, mix thoroughly, and react at room temperature for 30 minutes;
[0085] (5) Add 500 nM F and react at room temperature for...
Embodiment 2
[0087] A copper ion detection kit based on click chemistry, comprising the following components:
[0088] (1) Nucleic acid sequences A, B, C, D, E, F, their sequences and corresponding modification groups are as follows:
[0089] A: 5'-NH 2 -CGAATCCTGAGT-N 3 -3' (SEQ ID NO: 1);
[0090] B: 5'-CH CH-CTACAAATACCTA-3' (SEQ ID NO: 2);
[0091] C: 5'-TAGGTATTTGTAGACTCAGGATTCG-3' (SEQ ID NO: 3);
[0092] E: 5'-TAGGTATTTGTAGGTTACACCCATGTTACTCT-3' (SEQ ID NO: 4);
[0093] D: 5'-GATATCAGCGATTAACACTCAGGATTCG-3' (SEQ ID NO: 5);
[0094] F: 5'-FAM-AGAGTATrAGGATATC-BHQ-3' (SEQ ID NO: 6);
[0095] (2) Sodium ascorbate solution;
[0096] (3) Hydroxyl-modified magnetic bead solution;
[0097] (4) 20 mM Tris-HCl buffer, pH 7.5, containing 100 mM NaCl and 15 mM MgCl 2 .
Embodiment 3
[0099] Detection of different concentrations of copper ions:
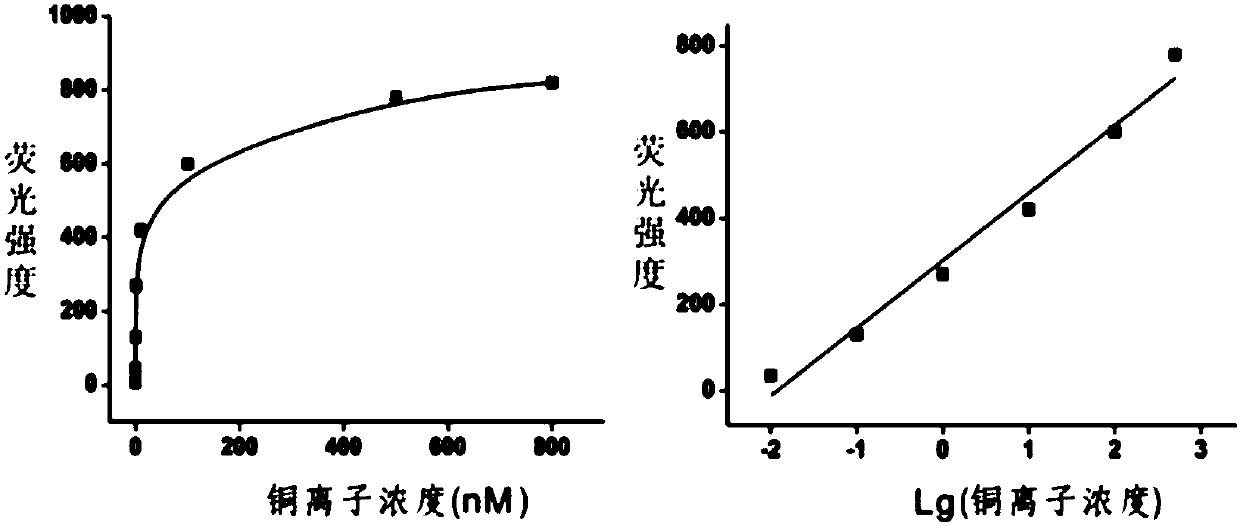
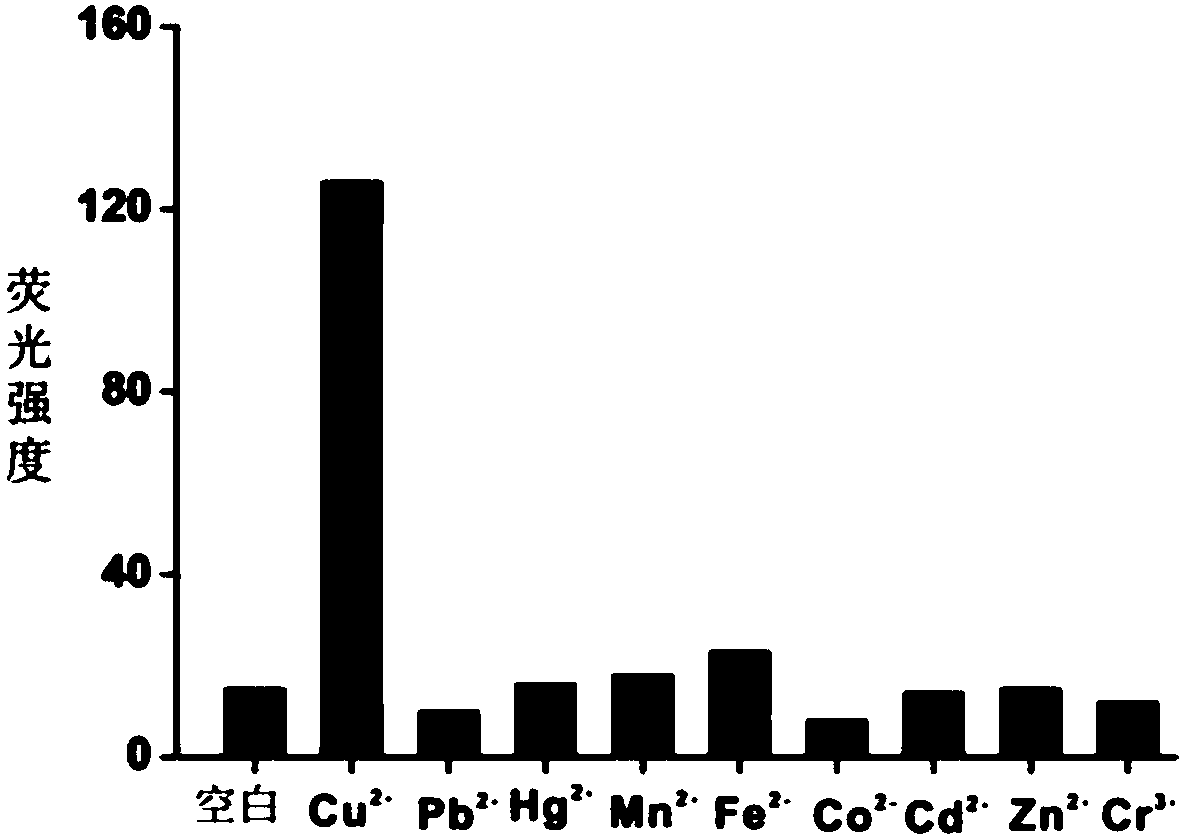
[0100] Prepare copper ion standard solutions with concentrations of 10 pM, 100 pM, 1 nM, 10 nM, 100 nM, 500 nM and 800 nM and store at room temperature. different concentrations of Cu 2+ Solution is added in the reaction system described in embodiment 1 respectively, detects fluorescence intensity after sufficient reaction, as figure 2 As shown, with Cu 2+ As the concentration increases, the corresponding fluorescence intensity gradually increases. When Cu2+ Saturation is gradually reached when the concentration exceeds 500 nM. Take Cu 2+ The logarithm of the concentration (lgC) is the abscissa, the fluorescence intensity is the ordinate, and the standard curve is drawn. The two have a good linear relationship, and the linear range is from 10 pM to 500 nM. The linear equation is: F = 302 +157 lgC (R 2 =0.975)( F is the fluorescence intensity, C is the copper ion concentration), according to the standard of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com