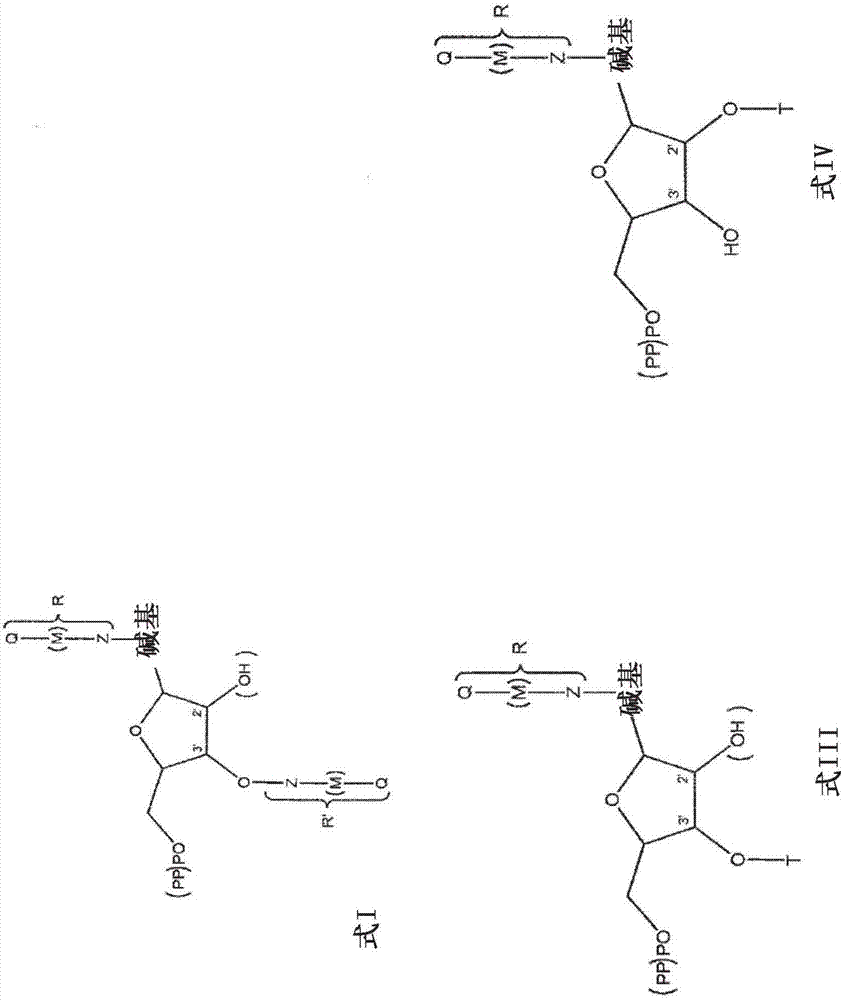
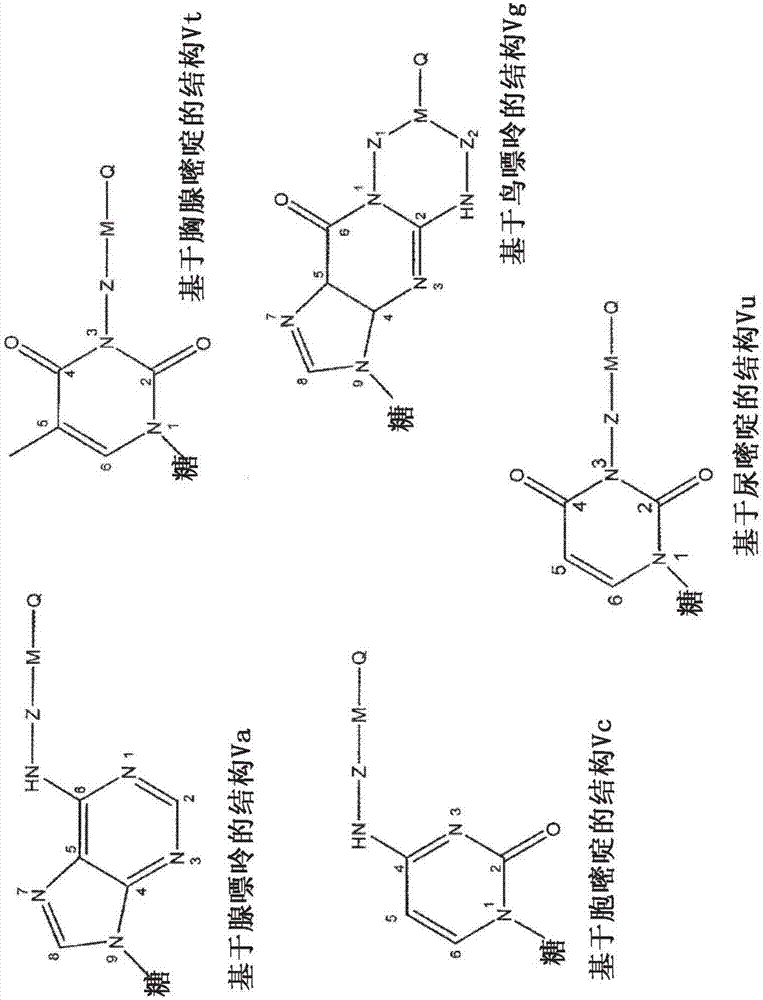
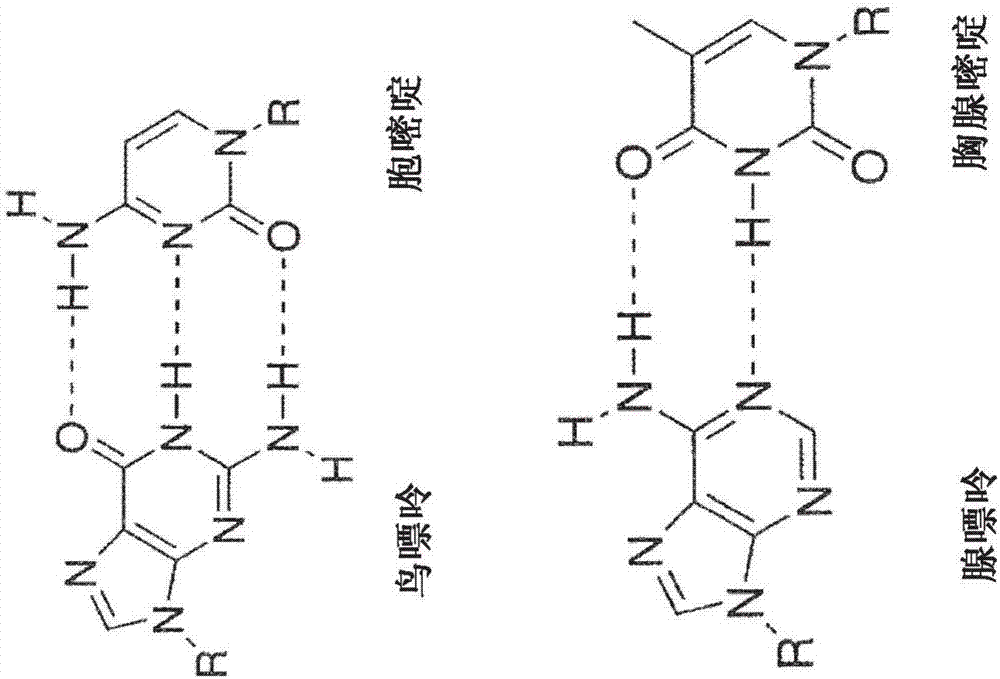
Modified nucleotides for synthesis of nucleic acids, a kit containing such nucleotides and their use for the production of synthetic nucleic acid sequences or genes
A nucleotide and nucleic acid technology, applied in the field of functionalized copolymer synthesis, can solve the problems of nucleic acid influence, poor use, and the inability of modified nucleotides to meet the expectations of enzymatic synthesis methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Example 1 – Compound NH 2 - Synthesis of dTTP-NitroB-Biot ( Figure 5 )
[0115] Step A1: To 5 g of 2'-deoxythymidine dissolved in pyridine add 2.2 ml of Et 3 N (triethylamine) and 175 mg DMAP (4-dimethylaminopyridine), then 5.25 g DMTCl (4,4'-dimethoxytrityl chloride) was added overnight at room temperature. Then add 2.4ml Et to the mixture 3N and 1.27ml MsCl (methanesulfonyl chloride). After incubating at room temperature for 2 h, the mixture was filtered and washed with ethyl acetate. The filtrate was concentrated and dissolved in 75 ml of ethanol, to which 1M NaOH was added. After reflux for 1.5 h, the mixture was cooled to room temperature and 1M HCl was added. Ethanol was evaporated in a rotary evaporator and the residue was washed with CH 2 Cl 2 extraction. After purification on a silica gel column, the product dTTP-A1 was obtained.
[0116] Step A2: To a solution of 2.237 mmol of the product dTTP-A1, 2.1 g of triphenylphosphine and 1.3 g of N-hydroxy...
Embodiment 2
[0121] Example 2 – Compound NH 2 - Synthesis of dGTP-NitroN-Biot ( Figure 6 )
[0122] Step B1: To a stirred solution of 1.845 mmol 2'-deoxyguanosine and 326 mg imidazole in anhydrous DMF was added 2.4 mmol tert-butyldimethylsilyl chloride. The reaction was incubated with stirring at room temperature for 20 h. The solvent was removed under vacuum and the residue was purified by chromatography to give the product dGTP-B1.
[0123] Step B2: To a solution of 2.237 mmol of the product dGTP-B1, 2.1 g of triphenylphosphine and 1.3 g of N-hydroxyphthalimide in 50 ml of tetrahydrofuran was added 1.75 ml of N,N'-di Isopropyl azodicarboxylate. After reheating overnight at room temperature, the reaction product was treated with 0.3 ml of water and the solvent was evaporated in vacuo. Most of the impurities were removed by chromatography to give the product dGTP-B2.
[0124] Step B3: 3.785 mmol of compound dGTP-B2 were dried several times using 10 ml of pyridine and evaporat...
Embodiment 3
[0128] Example 3 – Synthesis of compound FA-Biot-dNTP ( Figure 7 )
[0129] Step C1: Mix 100 μl of 1M ω1-biotin nonanoic acid in DMF with 100 μl of 1M carbonyldiimidazole in DMF. The formation of the imidazolium ion occurs within 30 s at room temperature. Then 100 µl of a 50 mM deoxyribonucleotide 5'-triphosphate aqueous solution was added to the mixture. The product formed within 12 h at room temperature. It is then precipitated with acetone and dissolved in water for final purification by chromatography to give the product FA-Biot-dNTP.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com