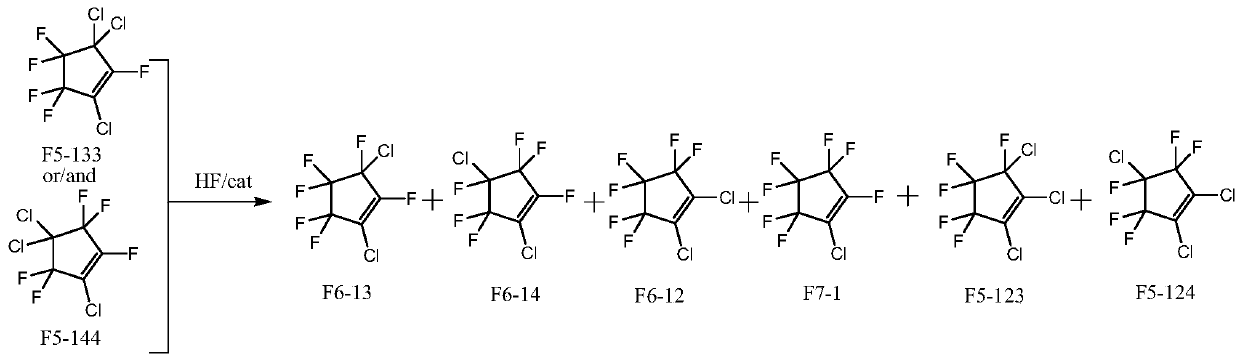
Method for simultaneously preparing dichlorohexafluorocyclopentene isomers
A technology for dichlorohexafluorocyclopentene and trichloropentafluorocyclopentene is applied in the field of simultaneous preparation of dichlorohexafluorocyclopentene isomers, and can solve problems such as being difficult to exist stably, unattractively, and difficult to find, etc. problem, to achieve the effect of stable activity and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of fluorination catalyst: Dissolve chromium nitrate in water, add precipitant ammonia water at 60°C, control the pH of the solution between 7.5 and 8.5, make it fully precipitate under stirring conditions, filter the formed slurry, and use Wash with ion water until neutral, then dry at 150°C for 12 hours to obtain chromium hydroxide. The above-mentioned chromium hydroxide and cobalt hydroxide are uniformly mixed according to the mass percentage of 95% and 5%, and press-molded to obtain a catalyst precursor, and then the catalyst precursor is roasted at 450 ° C for 10 hours under a nitrogen atmosphere. Activate at 300°C for 10 hours with a mixed gas consisting of hydrogen fluoride and hydrogen at a molar ratio of 10:1 to prepare a fluorination catalyst.
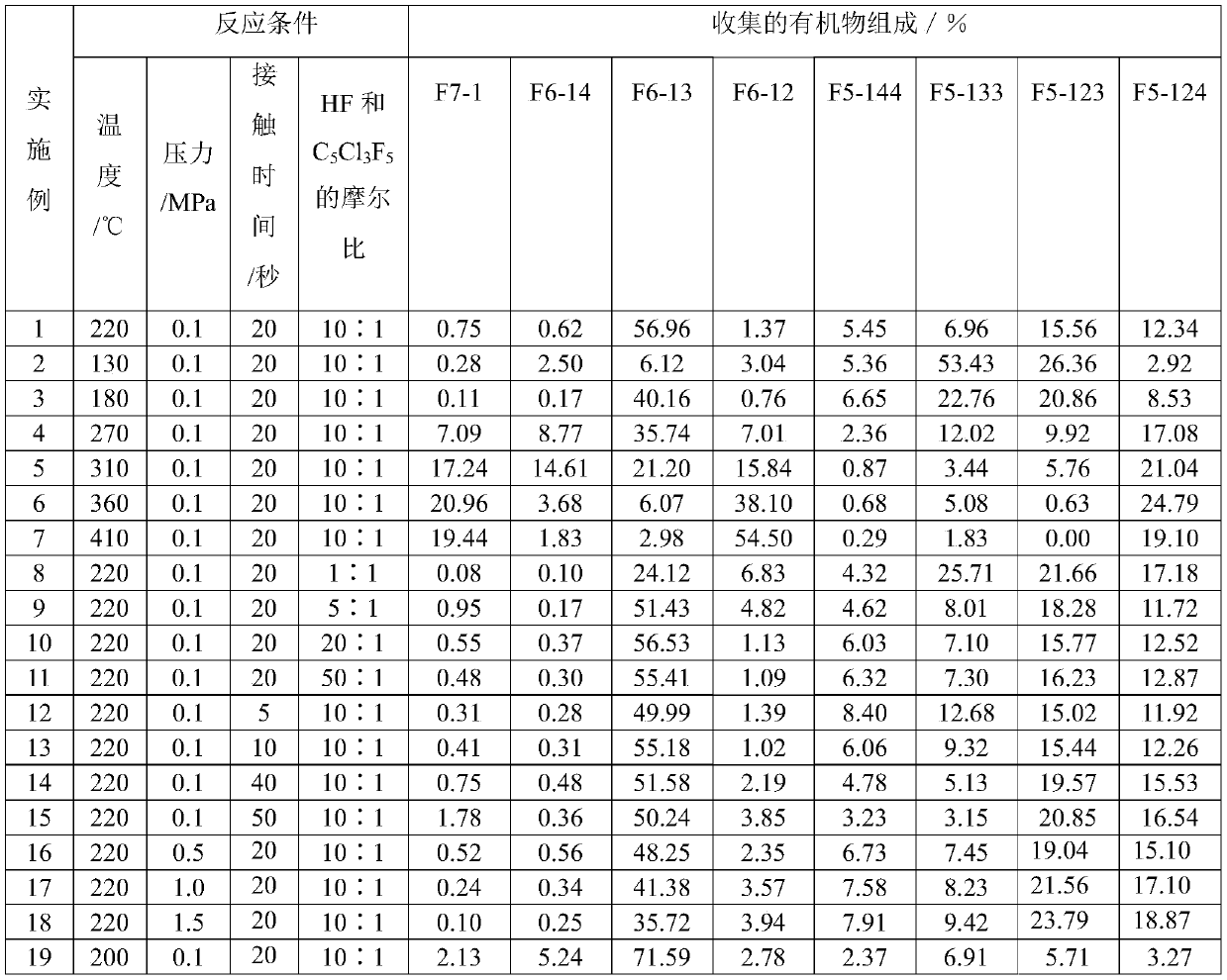
[0031] A tubular reactor made of Inconium alloy with an inner diameter of 1 / 2 inch and a length of 30 cm was filled with 10 ml of the above-prepared fluorination catalyst. The temperature of the reactor is ra...
Embodiment 2
[0033] The same operation as in Example 1, except that the reaction temperature was changed to 130° C., the results are shown in Table 1.
Embodiment 3
[0035] The same operation as in Example 1, except that the reaction temperature was changed to 180°C, the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com