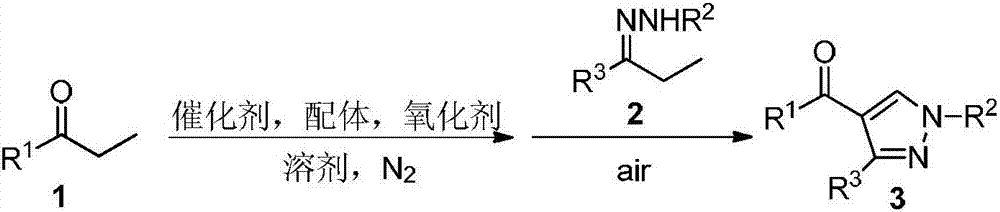
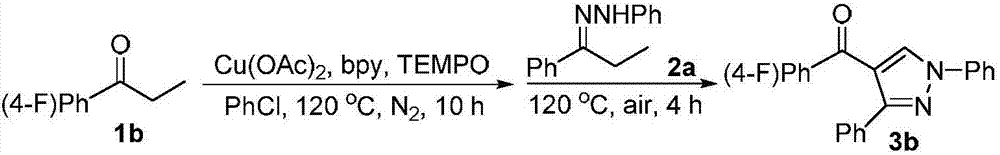
Method for synthesizing 4-acyl pyrazole compound from non-cyclic ketone hydrazone
A technology for acylpyrazoles and acyclic ketone hydrazones, which is applied in the field of organic synthesis, can solve the problems of limited method of 4-acyl-substituted pyrazoles, poor regioselectivity, and difficulty in obtaining raw materials, avoiding purification treatment and easy operation. , a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011]
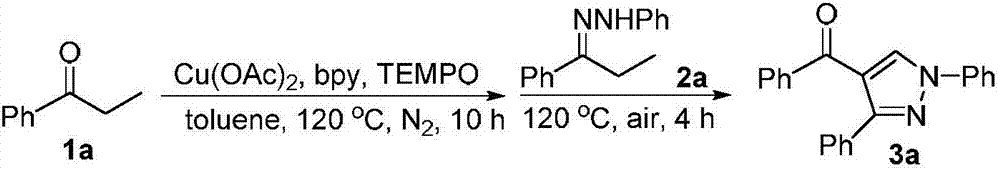
[0012] Add 1a (0.5mmol, 67mg), copper acetate (Cu(OAc) 2 , 0.05mmol, 9mg), 2,2'-bipyridine (bpy, 0.05mmol, 8mg), 2,2,6,6-tetramethylpiperidine nitrogen oxide (TEMPO, 0.5mmol, 78mg) and toluene ( toluene, 3mL), the reaction tube was sealed after evacuated and filled with nitrogen, and placed in an oil bath at 120°C to stir for 10 h. Then 2a (0.5 mmol, 112 mg) was added to the reaction system, and the stirring reaction was continued in an oil bath at 120° C. for 4 h in an air atmosphere. The reaction was quenched by adding 10 mL of water, extracted with ethyl acetate (10 mL×3), and then the organic phase was washed with water and saturated brine in sequence, and dried over anhydrous sodium sulfate. Filtration, spin-drying, and silica gel column separation (petroleum ether / ethyl acetate=20 / 1) gave the white solid product 1,3-diphenyl-4-benzoylpyrazole 3a (68 mg, 42%). The characterization data of this compound are as follows: 1 H NMR (400MHz, CDCl 3 )δ:7.32-7.42(m...
Embodiment 2
[0014] Add 1a (0.5mmol, 67mg), Cu(OAc) 2 (0.1mmol, 18mg), bpy (0.05mmol, 8mg), TEMPO (0.5mmol, 78mg) and toluene (3mL), vacuumize and fill the reaction tube with nitrogen, seal the reaction tube, and place it in an oil bath at 120°C for 10h with stirring. Then 2a (0.5 mmol, 112 mg) was added to the reaction system, and the stirring reaction was continued in an oil bath at 120° C. for 4 h in an air atmosphere. The reaction was quenched by adding 10 mL of water, extracted with ethyl acetate (10 mL×3), and then the organic phase was washed with water and saturated brine in sequence, and dried over anhydrous sodium sulfate. Filtration, spin-drying, and silica gel column separation (petroleum ether / ethyl acetate=20 / 1) gave white solid product 1,3-diphenyl-4-benzoylpyrazole 3a (97 mg, 60%).
Embodiment 3
[0016] Add 1a (0.6mmol, 80mg), Cu(OAc) 2 (0.1mmol, 18mg), bpy (0.05mmol, 8mg), TEMPO (0.5mmol, 78mg) and toluene (3mL), vacuumize and fill the reaction tube with nitrogen, seal the reaction tube, and place it in an oil bath at 120°C for 10h with stirring. Then 2a (0.5 mmol, 112 mg) was added to the reaction system, and the stirring reaction was continued in an oil bath at 120° C. for 4 h in an air atmosphere. The reaction was quenched by adding 10 mL of water, extracted with ethyl acetate (10 mL×3), and then the organic phase was washed with water and saturated brine in sequence, and dried over anhydrous sodium sulfate. Filtration, spin-drying, and silica gel column separation (petroleum ether / ethyl acetate=20 / 1) gave the white solid product 1,3-diphenyl-4-benzoylpyrazole 3a (105 mg, 65%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com