Antibacterial peptide PR39 and PG1 co-expression vector and method for preparing PR39 and PG1 transgenic mice
A technology of co-expression vector and antibacterial peptide, applied in the field of genetic engineering, can solve the problem of transgenic animals that do not co-express two antibacterial peptides, and achieve the effect of reducing the use of antibiotics and solving food safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
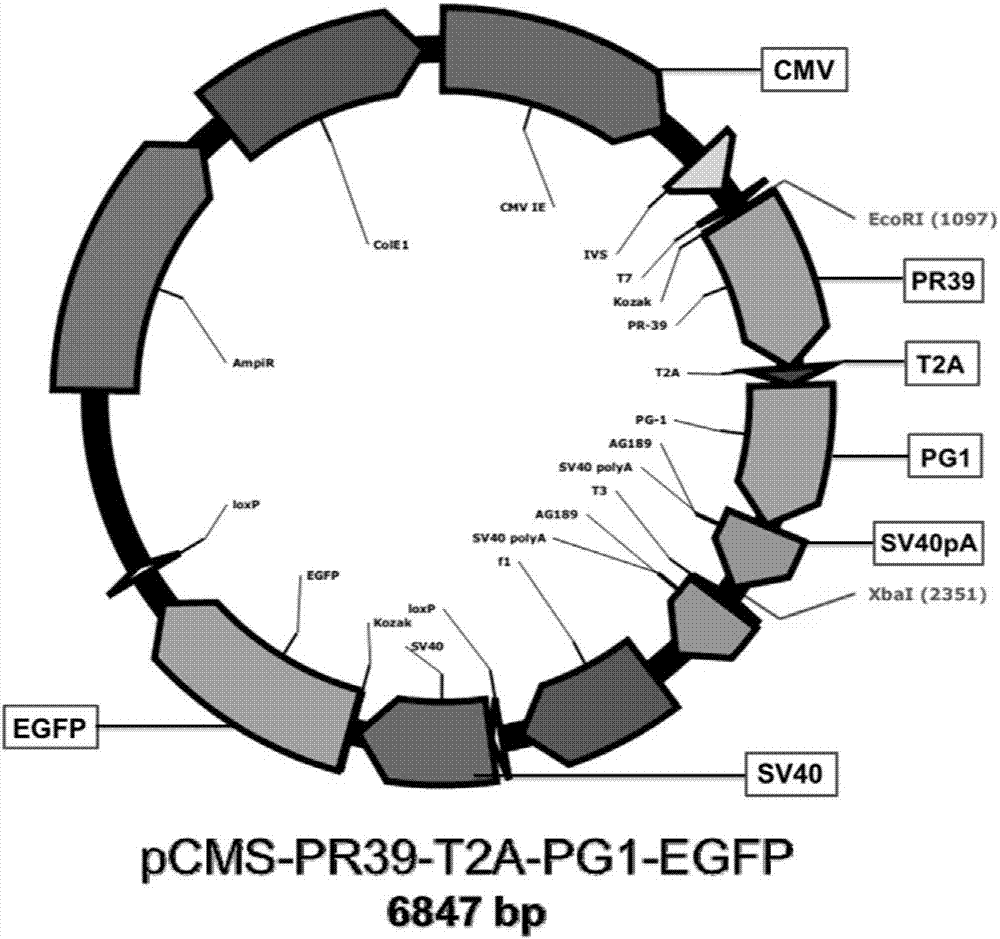
[0050] Example 1: Construction of eukaryotic co-expression vector pCMS-PR39-T2A-PG1-EGFP
[0051] (1) Co-expression vector construction strategy
[0052] The two antimicrobial peptide genes PR39 and PG1 were connected in series through a T2A linking peptide, and then cloned into the eukaryotic expression vector pCMS-EGFP with two independent expression cassettes regulated by the two promoters (purchased from Youbao Biotechnology Co., Ltd.) Above, the co-expression of two antimicrobial peptide genes is regulated by the CMV promoter alone, and the sequence of the elements is CMV-Kozak-PR39-T2A-PG1-SV40pA; the expression of the EGFP gene is regulated by the SV40 promoter alone, and the sequence of the elements is arranged is SV40-Kozak-EGFP-bGHpA. The two expression systems are relatively independent and complete without interfering with each other, and can achieve the purpose of simultaneously and efficiently expressing two target proteins and one marker protein on the same euk...
Embodiment 2
[0064] Example 2: Large-scale extraction and enzyme digestion identification of PR39 and PG1 co-expression vector without endotoxin
[0065] (1) In order to reduce the cytotoxicity of the plasmid transfection to 293 cells, a large amount of co-expression vectors were extracted using OMEGA’s endotoxin removal kit Endo-free Plasmid Mini Kit before transfection. The operation steps are detailed in the instruction manual.
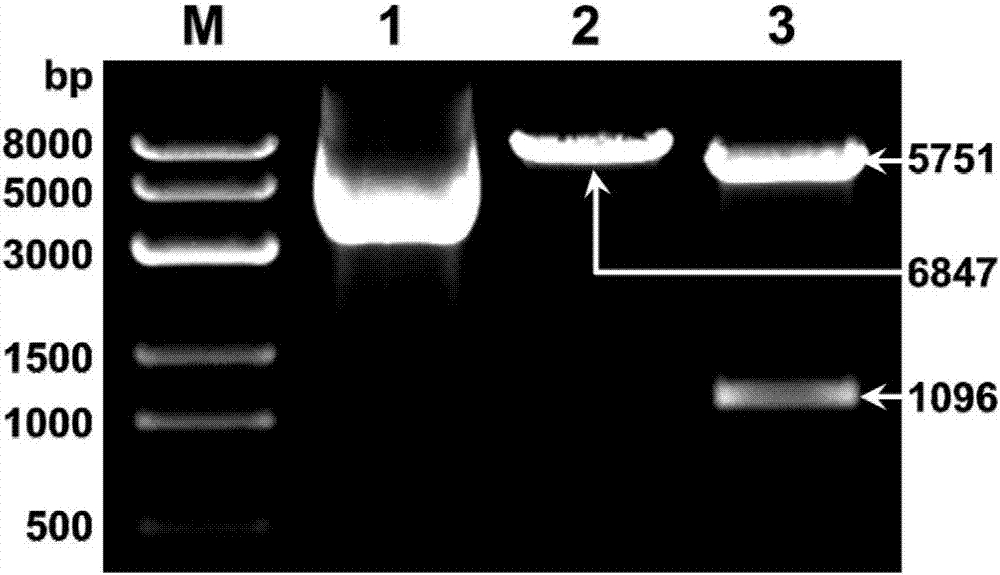
[0066] (2) Enzyme digestion identification of PR39 and PG1 co-expression vector
[0067] The extracted plasmids were identified by single- and double-enzyme digestion using FastDigest rapid restriction endonucleases.
[0068] The results showed that after a large amount of co-expression vectors were extracted, linearization (6847bp) was carried out at a single site (2368) of the vector with restriction endonuclease Not I, and two sites of the vector with restriction endonucleases Xho I and Bgl II were A single site (1092 and 6843) was double digested, cut into...
Embodiment 3
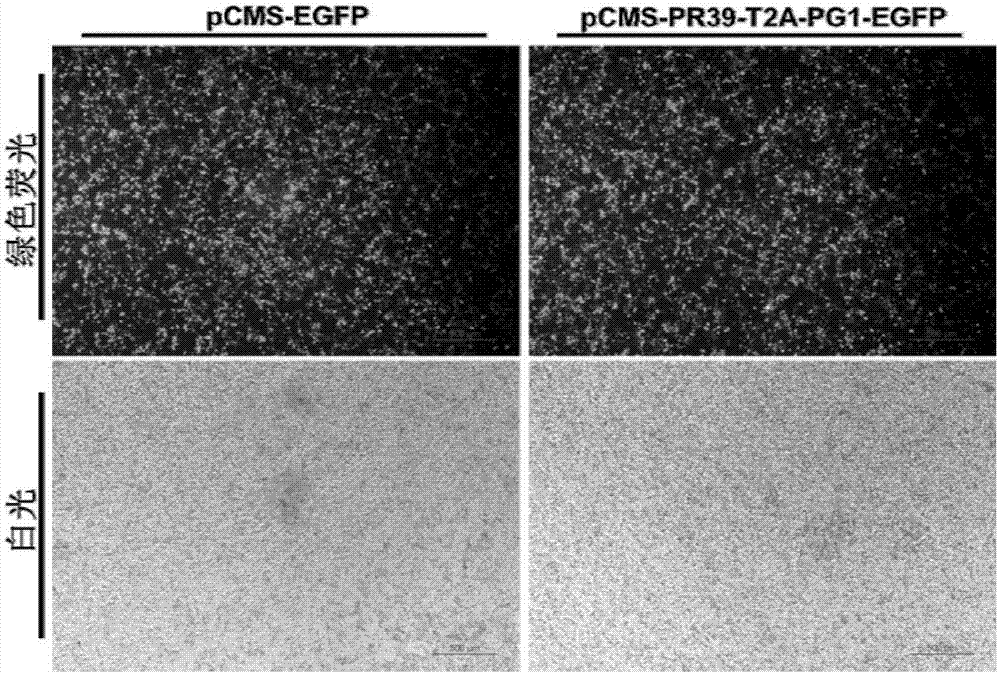
[0069] Example 3: PR39 and PG1 co-expression vector transfected 293 cells
[0070] The specific operation of PR39 and PG1 co-expression vector transfection into 293 cells is as follows:
[0071] 1. Cells were subcultured evenly in a 6-well plate after thawing, cultured with 10% FBS complete medium, and transfected when the cells grew to a confluence of 70% to 90%;
[0072] 2. In a separate test tube, dilute 7.5 μL Lipofectamine 3000 and 7.5 μg plasmid DNA in 125 μL Opti-MEM, add 15 μL P3000 reagent to the diluted DNA and mix well;
[0073] 3. Mix the diluted plasmid DNA with the diluted Lipofectamine 3000 reagent 1:1, and incubate at room temperature for 5 minutes;
[0074] 4. Carefully and evenly add the above mixture to the cells containing 1% double antibody and 10% FBS complete medium, avoid blowing up the 293 cells that are not firmly attached, gently cross-mix and put the cells back to 37°C , 5% CO2, and saturated humidity in a cell incubator to continue culturing;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com