Method for preparing zinc ferrite
A zinc ferrite, zinc iron technology, applied in chemical instruments and methods, inductance/transformer/magnet manufacturing, magnetic objects, etc., can solve the problem of long processing time, small product particle size, reaction temperature and oxidation time. In order to achieve the effect of high comprehensive utilization of resources, improved economic benefits of enterprises, and convenient source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
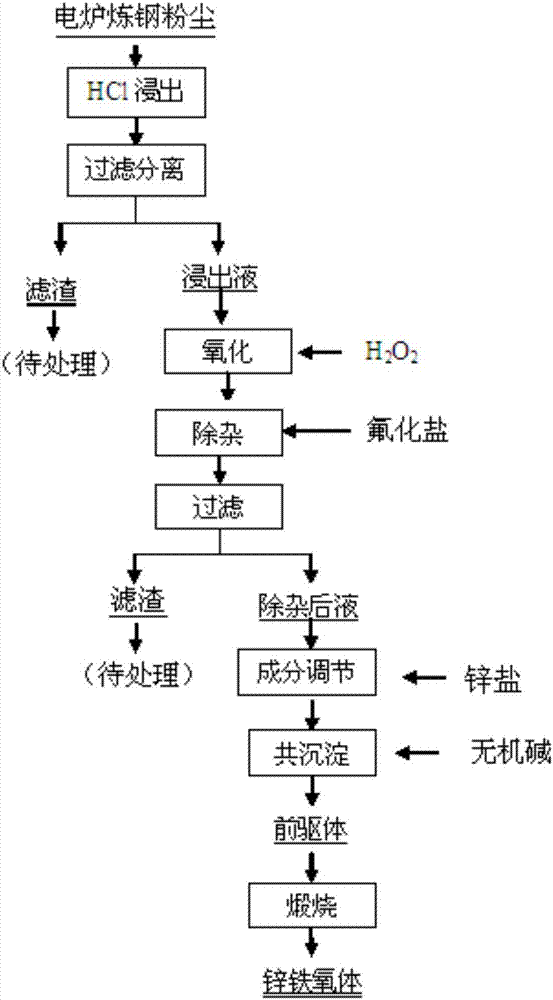
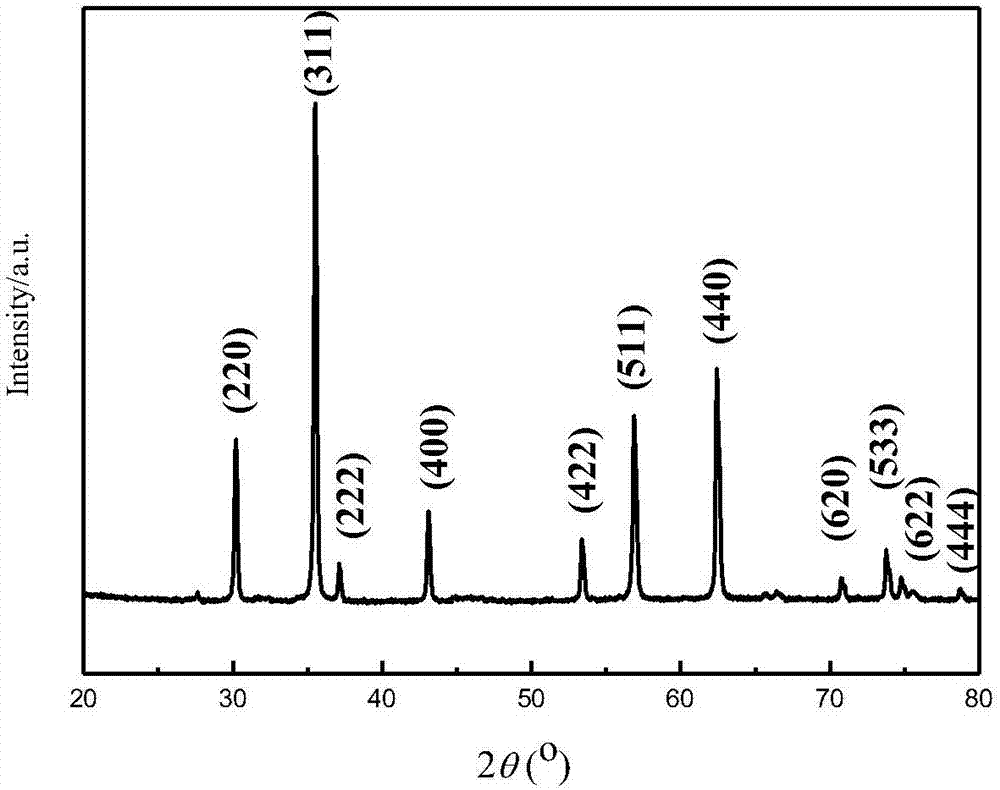
[0037] according to figure 1 As shown, take 10g of the electric furnace steelmaking dust, add 4mol / L HCl solution to it, stir and leach for 2 hours at a liquid-solid ratio of 4:1 at 60°C, filter, press H 2 o 2 with Fe 2+ Add H to the filtrate at a molar ratio of 2:1 2 o 2 , after reacting for 0.5 hours, NaF was mixed according to the concentration ratio of the substances in the solution to F + : Ca 2+ =2.5:1.0 Add to the above oxidized liquid to purify and remove impurities, keep the temperature at 60°C, stir for 1 hour, after filtering, add ZnCl to the filtrate 2, control the concentration ratio of zinc and iron in the purified liquid to Zn:Fe=0.6:1.0, add NaOH solution, adjust the pH value of the solution to 11, keep the temperature at 75°C, stir for 1 hour, filter and wash the zinc-containing After the iron precipitate was dried, it was calcined at 700°C for 6 hours to obtain 4.23g of zinc ferrite powder product, the composition of which was Zn 27.09, Fe 46.16, O 26.7...
Embodiment 2
[0039] according to figure 1 As shown, take 10g of the electric furnace steelmaking dust, add 3mol / L HCl solution to it, stir and leaching for 2.5 hours at a liquid-solid ratio of 5:1 at a temperature of 70°C, filter, press H 2 o 2 with Fe 2+ Add H to the filtrate at a molar ratio of 2:1 2 o 2 , after 0.5 hours of reaction, the NH 4 F according to the concentration ratio of the substance in the solution F + : Ca 2+ =3.5:1.0 is added to the above-mentioned oxidized liquid to purify and remove impurities, keep the temperature at 50°C, stir for 1.5 hours, after filtering, add ZnO to the filtrate, and control the concentration ratio of zinc and iron in the purified liquid to Zn: Fe=0.55:1.0, add NH 3 ·H 2 O solution, adjust the pH value of the solution to 9.5, keep the temperature at 65°C, stir for 1.5 hours, filter and wash the zinc-containing iron precipitate, after drying, calcinate at 600°C for 8 hours to obtain 4.05g of zinc ferrite powder The composition of the prod...
Embodiment 3
[0041] according to figure 1 As shown, take 10g of the electric furnace steelmaking dust, add 3.5mol / L HCl solution to it, stir and leaching for 1.5 hours at a liquid-solid ratio of 7:1 at a temperature of 50°C, filter, press H 2 o 2 with Fe 2+ Add H to the filtrate at a molar ratio of 2:1 2 o 2 , after reacting for 0.5 hours, KF is compared to F by the concentration ratio of substances in the solution + : Ca 2+ =3.0:1.0 Add to the above oxidized liquid to purify and remove impurities, keep the temperature at 70°C, stir for 1 hour, after filtering, add ZnCO to the filtrate 3 , the concentration ratio of the zinc-iron substance in the purified liquid is controlled at Zn:Fe=0.5:1.0, adding Na 2 CO 3 Solution, adjust the pH value of the solution to 8, keep the temperature at 75°C, stir for 1 hour, filter and wash the zinc-containing iron precipitate, after drying, calcinate at 650°C for 7 hours to obtain 4.36g of zinc ferrite powder product , its composition by mass perce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com